Registration of cosmetics in Ukraine is one of the popular queries in search engines. This is not surprising, because the trend of the last decade is the growth of the cosmetic market. This is indicated by a stable increase in the volume of production and sale of cosmetic products. Cosmetics have long become not just a product for care, but also a necessary element of a person’s daily use.
The Ukrainian market of cosmetic products is no exception. Therefore, the issue of the appearance of new products, increasing the number of manufacturers and technical regulation of the circulation of cosmetics is an urgent issue for Ukrainian legislation.
How to legally register cosmetic products in Ukraine?
Is the certification of cosmetics mandatory, and when does the new Technical Regulation come into effect? Vitaliy Kovbasa, the chief lawyer of MIASPHERA company, answers these and other questions.
We will provide some theory and understanding of the term “cosmetic products” from the Ukrainian legislation.
Cosmetic products in Ukraine are regulated in accordance with the Law On Cosmetic Products dated 04/05/2012 No. 4616-VI.
Any substance or mixture of substances can be considered a cosmetic product. They are intended for use on the external parts of the human body in order to clean, aromatize, restore, protect and correct the smell.
Every foreign manufacturer or importer that brings cosmetic products to the market of Ukraine undergoes a simplified procedure for declaring compliance. Cosmetics from the EU already meet the standards that will soon be implemented in our country. It is enough for the importer to provide a certificate from the manufacturer and a safety data sheet for the products.
The national producer has to do this procedure by obtaining a conclusion from a sanitary-epidemiological examination of production and products.
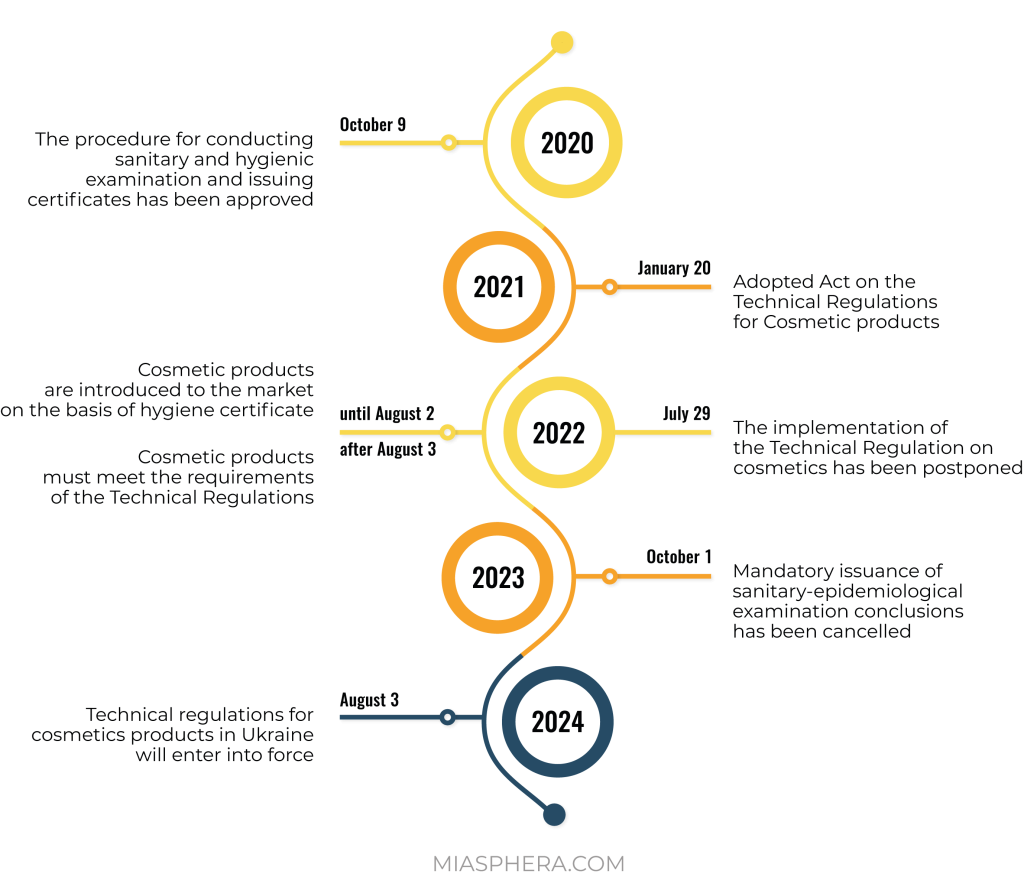
Registration of cosmetic products in Ukraine for a national manufacturer is carried out through the voluntary receipt of a sanitary-hygienic expert opinion, which confirms the safety of products for human health and gives the right to sell them. This procedure is valid until the Technical Regulation is fully implemented.
The process of regulation of cosmetic products in Ukraine is actively integrated with the legislation of the European Union. On the basis of EU Regulation No. 1223/2009, a technical regulation for cosmetic products was developed for Ukraine. This normative act was postponed several times, but was still adopted by the Cabinet of Ministers on January 20, 2021.
In connection with the hostilities, the Ministry of Health proposed postponing the entry into force of the Technical Regulation until August 3, 2024, and introduced a transition period for participants in the cosmetic products market until August 3, 2026.
The main purpose of introducing the Technical Regulation is to establish new standards for cosmetic products on the market of Ukraine, to define the rights and obligations of market participants regarding the introduction of cosmetic products into circulation, as well as to remove administrative barriers in trade with the countries of the European Union.
The process of registration of cosmetic products will become radically new after the entry into force of the technical regulation.

It should be noted that there is a transition period until the entry into force of the Technical Regulation. This gives market operators time to prepare. The sale of cosmetics manufactured before 08/03/2026 will not be prohibited or restricted for the next 5 years.
If you are a manufacturer of cosmetics and are planning further development of your own production, you should approach this issue strategically now. The transition period in legislation is always a time of additional actions and new steps. In order not to waste time and avoid a number of unfortunate mistakes when registering cosmetic products under the new rules – contact MIASPHERA! We will provide full legal support and support at all stages of bringing cosmetic products to the market of Ukraine! MIASPHERA specialists will gladly share their successful experience!
What Miasphera can do for a cosmetics manufacturer:
We are always one step ahead and work according to new standards!
Would you like to receive a consultation? Leave an application using the link.
If you are a manufacturer or exporter of cosmetic products and have a desire to conquer the market of the European Union countries, you must do the registration procedure for your cosmetic products.
The procedure goes through the assessment of the conformity of cosmetic products in accordance with EU regulations and directives. If say briefly: your product must meet EU standards for this type of product.
Requirements for cosmetic products on the EU market are regulated by Regulation №1223/2009.
The regulation prohibits cosmetic products whose ingredients or final composition have been tested on animals from entering the EU market. Carcinogenic, mutagenic or toxic substances for reproductive health are also prohibited for use.
Conformity assessment consists of two main procedures: drawing up a Product Safety Report (CPSR) and notification. It sounds scary, but in this article we will explain this confusing issue.
Cosmetic products include a wide range of products. Direct contact with the human body is one common feature. It is this feature that is the reason for careful study of the physicochemical parameters of any cosmetic product.
A Safety Report (CPSR) allows you to make sure that the product under investigation is safe for use. It contains important data on the results of research on the composition and individual components. The procedure includes the creation of an information file, where each tool is marked with the appropriate marking and conditions of production compliance with EU legislation.
Notification is the process of entering cosmetic products into the pan-European database (CPNP). This process based on the basis of a product safety report.
So, we’re having dealt with the definition of the main terms! Now we can form the stages on the way to a successful procedure for evaluating the conformity of cosmetic products:

Successful completion of the first stage allows you to create a road map of the entire process of product registration and the final list of necessary documents for the creation of a dossier – PIF file.
In the case of registration of cosmetic products on the territory of the European Union countries, it is necessary to take care of the presence of an authorized representative.
This responsible person must register on the territory of the EU country, has full information about the products, is legally responsible for compliance with the requirements of cosmetics regulations and, if necessary, provides all necessary information at the request of supervisory authorities for control and monitoring of activities.
According to the Cosmetic Regulation (EU) №1223/2009, the authorized representative is legally responsible for a cosmetic product of a non-European manufacturer on the EU market.
All cosmetic products entering the EU market must have the following package of documents:

The Safety Data Sheet (MSDS) of cosmetic products is developed based on the data of the manufacturer and the authorized person. It contains more detailed information about: composition, possible risks of harm to humans, rules of conduct in case of improper use, storage and disposal conditions. This document is important not only for export operations, but also for the end user to ensure the correct and safe use of the product.
Registration may be refused if the cosmetics do not comply with the established safety standards of a certain country or if an incomplete package of documents is submitted.