Batch certification is one of the main legal procedures. It can be applied for registration of imported medical devices in Ukraine.
The certificate of conformity for the batch is issued without experts going to the place of production. In fact, it is issued on the basis of a valid technical file and inspection of the lot by an auditor at customs.
Therefore, the procedure for carrying out the procedure is reduced to:
– acquaintance with the technical file;
– preparation of instructions and labeling under Ukrainian legislation;
– drawing up a contract for the customer or Authorized Representative;
– audit of the batch directly at customs;
– certificate issuance (approximately 3 – 5 days);
– customs clearance as a medical product (with a tax rate of 7%).
Obtaining a certificate of conformity for a batch of medical goods is economically feasible if the products are not imported systematically or once. As a rule, this is the supply of artificial medical electrical equipment or medical products of the same type (one model and one brand).
Would you like to learn about the possibility of batch certification of medical products?
Order a preliminary consultation from Miasphera by +38 068 498 88 58
There is traditionally a wide range of dietary supplements on the market of Ukraine. They cover many directions of physiological action and have different forms of release. But sometimes, the information on the package about the properties of dietary supplements can confuse consumers, especially if these products were bought in pharmacies.
Therefore, the manufacturer of dietary supplements should pay special attention to labeling. According to the requirements of Ukrainian legislation, all mandatory information about the product must be provided directly on the package or label.
So, a correctly labeled dietary supplement for the manufacturer is not only a powerful incentive to enable the consumer to make an informed choice without misleading him, but also a clear compliance with the requirements of Ukrainian legislation and a minimization of appeals from control authorities.
Understanding the intricacies of medical device certification can seem difficult even for those who have already encountered this topic many times. Therefore, we are sharing useful information and considering the main aspects and issues related to the certification of medical devices on the territory of Ukraine.
Let’s go!
Certification of medical devices is a process of assessing the compliance of a medical device with the requirements of the Technical Regulations. Certification confirms the safety and effectiveness of medical devices used by humans.
Of course! Compliance assessment of medical devices is a legal requirement. This confirms the legal introduction of medical products into circulation on the territory of Ukraine and their subsequent sale.
The responsibility for declaring the medical product lies directly with the manufacturer or an authorized person, in case the medical products are imported from other countries. The declaration is made under the full responsibility of the manufacturer.
The procedure for evaluating the conformity of medical devices depends on the type and safety class of the medical device. The main procedures are self-declaration (for medical devices of the I-risk class, non-sterile, without a measurement function, for in vitro) and assessment of conformity through the manufacturer’s audit (I-sterile, with a measurement function, IIa, IIb, III).
In some cases, the batch certification or the procedure with EU recognition of the certificate is used.
The centers of regulation and supervision are the Ministry of Economic Development and Trade, the Ministry of Health and the State Medical Service of Ukraine.
Assessment of the conformity of medical products in Ukraine is carried out by specially accredited conformity assessment organizations of state or private ownership. The current list of accredited organizations is published by the Ministry of Economic Development and Trade.
Our experts accompany the manufacturer at all stages of the conformity assessment procedure, starting from consultation and collecting documents and ending with obtaining expert opinions. We also provide full legal support and Authorized Representative services.
Have additional questions? Leave a request for a consultation or call us.
We are always in touch +38 068 498 88 58
Have you thought about who is responsible for the quality and safety of imported goods? In Ukraine, this role belongs to the Authorized Representative. This is a legal entity or an individual entrepreneur who has residency.
The presence of an authorized representative for any non-resident manufacturer is a requirement of Ukrainian legislation. Therefore, one of the first steps in the process of certification of medical products or cosmetics is the appointment of the authorized representative of the manufacturer in Ukraine. The authorized representative acts as an official intermediary between the producer and the Ukrainian market, including with state institutions responsible for market surveillance.
An Authorized Representative has certain duties. These duties are defined by the Technical Regulations and legislative acts:
Usually, foreign manufacturers choose an authorized representative according to their own requirements and preferences. This can be an existing distributor or an independent company such as Miasphera.
Let’s consider both cases in more detail.
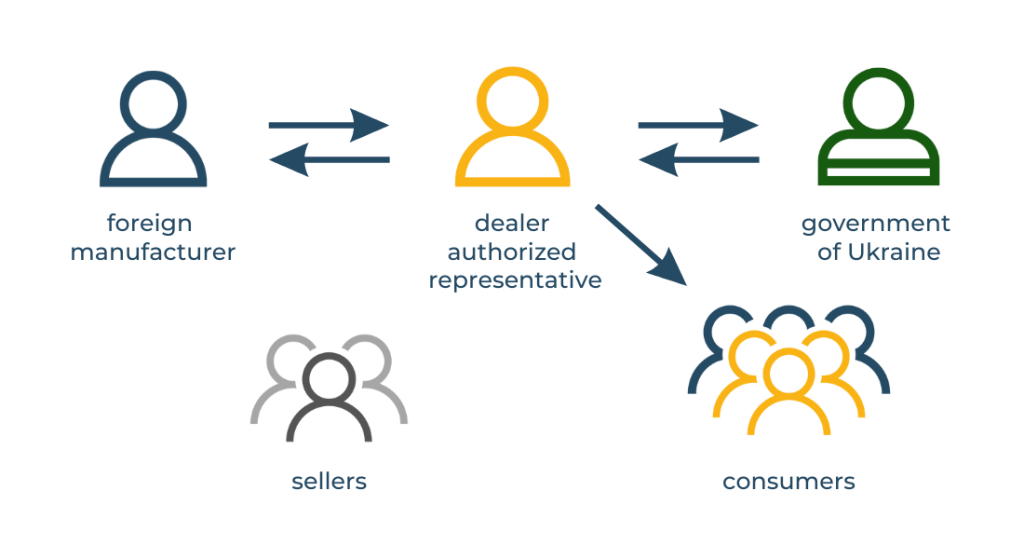
If the authorized representative is a dealer-importer, the manufacturer is more dependent on its conditions. In this case, the representative-dealer often acts in his own interests, closing the supply and sales to himself and limiting the rights of the dealership to other companies. In addition, he has an additional competitive advantage – his name, as an Authorized Representative, is present on the labeling or on the instructions of all products.

If the Authorized Representative is a company that is not involved in the sale of products, the manufacturer can independently choose any number of dealers with whom he wants to cooperate.
Important clarification! Each type of medical device must be associated with only one Authorized Representative.
As you can see, the last option has more advantages for the manufacturer, as it allows you to regulate the number of dealers and grant the right to import and distribute your products to other companies.
Miasphera offers independent outsourcing services of the Authorized Representation, because we have many years of experience.
We work with a wide range of medical, cosmetic, food and veterinary products and always keep our finger on the pulse of legislative innovations.
Contact us and take an important step towards a successful start on the Ukrainian market!
Medical devices are classified depending on the design, features of application and potential danger in case of their incorrect use. Therefore, before starting the certification process, the manufacturer of the medical device must determine its medical purpose and intended use.
Each country has its own requirements. Currently, the medical device classifier NC 024:2023 operates in Ukraine. It is created on the basis of the GMDN international nomenclature.
The safety class of a medical product depends on the potential risk during its use by the consumer. Medical products are classified according to the following criteria: invasiveness, duration of use, presence of contact with the human body, effect on vital human organs, as well as the possibility of using energy sources together with the product.
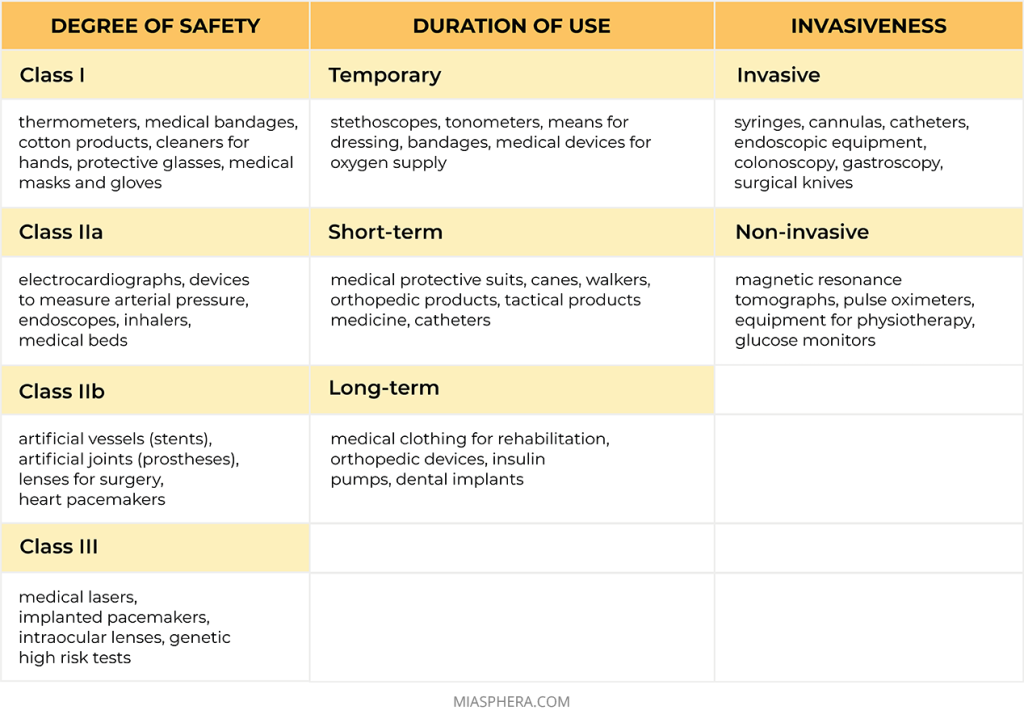
So, according to the degree of safety, medical products are divided into:
Class I — a low proportion of risk
Class IIa — the average proportion of risk
Class IIb — increased risk
Class III — high proportion of risk
According to the duration of use, medical products are:
Temporary – for continuous use up to 60 minutes;
Short-term – for continuous use up to 30 days;
Long-term – for continuous use for a period of 30 days or more.
According to invasiveness, medical products are:
Non-invasive are medical devices or means that do not require invasion or penetration into the human body for diagnosis, monitoring or treatment.
Invasive are medical products that are completely or partially introduced into the human body through its surface or body opening.
Accepted safety classes of medical devices reflect the potential risk in case of their use by consumers. Therefore, depending on the defined class and characteristics, the medical device certification procedure may differ. After all, the higher the risk class, the more complicated the registration procedure is.
In the further sale of products, mistakes in determining the class can be expensive. Therefore, if you have doubts about the correct classification of your own product, contact Miasphera specialists!
We will be happy to provide comprehensive advice and suggest the best certification options for your product.
Leave an application for consultation.
Registration of cosmetics in Ukraine is one of the popular queries in search engines. This is not surprising, because the trend of the last decade is the growth of the cosmetic market. This is indicated by a stable increase in the volume of production and sale of cosmetic products. Cosmetics have long become not just a product for care, but also a necessary element of a person’s daily use.
The Ukrainian market of cosmetic products is no exception. Therefore, the issue of the appearance of new products, increasing the number of manufacturers and technical regulation of the circulation of cosmetics is an urgent issue for Ukrainian legislation.
How to legally register cosmetic products in Ukraine?
Is the certification of cosmetics mandatory, and when does the new Technical Regulation come into effect? Vitaliy Kovbasa, the chief lawyer of MIASPHERA company, answers these and other questions.
We will provide some theory and understanding of the term “cosmetic products” from the Ukrainian legislation.
Cosmetic products in Ukraine are regulated in accordance with the Law On Cosmetic Products dated 04/05/2012 No. 4616-VI.
Any substance or mixture of substances can be considered a cosmetic product. They are intended for use on the external parts of the human body in order to clean, aromatize, restore, protect and correct the smell.
Every foreign manufacturer or importer that brings cosmetic products to the market of Ukraine undergoes a simplified procedure for declaring compliance. Cosmetics from the EU already meet the standards that will soon be implemented in our country. It is enough for the importer to provide a certificate from the manufacturer and a safety data sheet for the products.
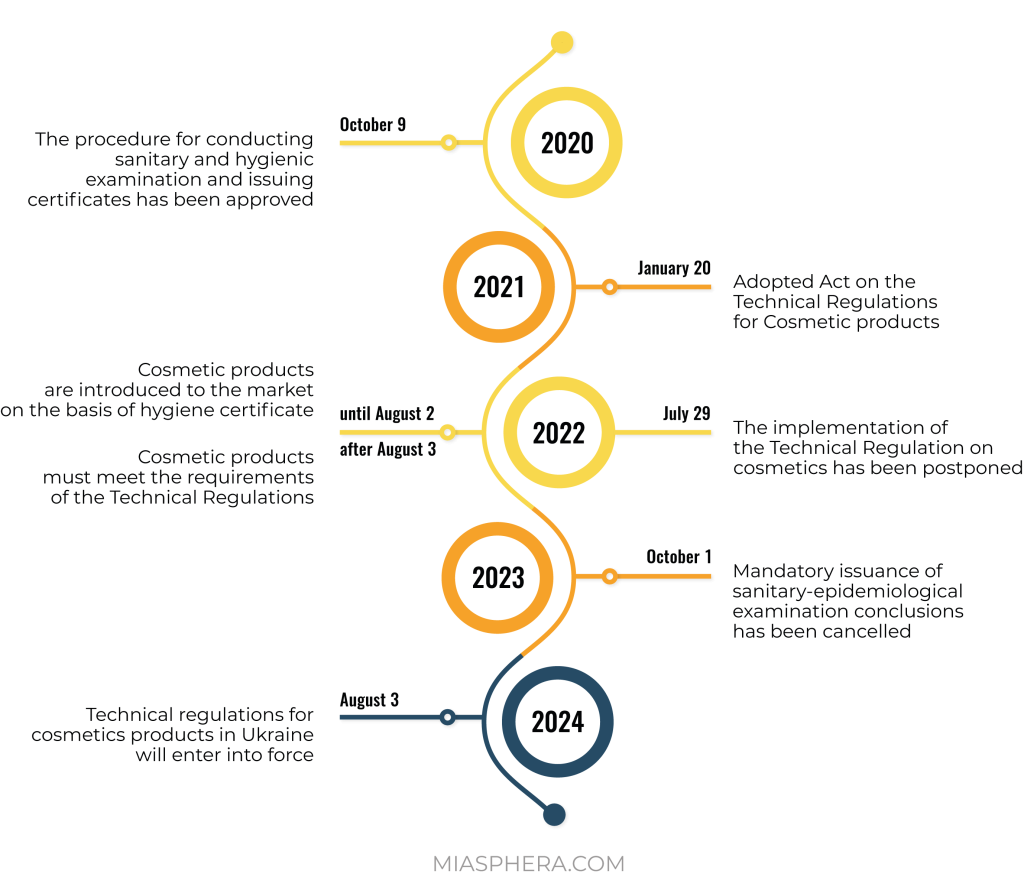
The national producer has to do this procedure by obtaining a conclusion from a sanitary-epidemiological examination of production and products.
Registration of cosmetic products in Ukraine for a national manufacturer is carried out through the voluntary receipt of a sanitary-hygienic expert opinion, which confirms the safety of products for human health and gives the right to sell them. This procedure is valid until the Technical Regulation is fully implemented.
The process of regulation of cosmetic products in Ukraine is actively integrated with the legislation of the European Union. On the basis of EU Regulation No. 1223/2009, a technical regulation for cosmetic products was developed for Ukraine. This normative act was postponed several times, but was still adopted by the Cabinet of Ministers on January 20, 2021.
In connection with the hostilities, the Ministry of Health proposed postponing the entry into force of the Technical Regulation until August 3, 2024, and introduced a transition period for participants in the cosmetic products market until August 3, 2026.
The main purpose of introducing the Technical Regulation is to establish new standards for cosmetic products on the market of Ukraine, to define the rights and obligations of market participants regarding the introduction of cosmetic products into circulation, as well as to remove administrative barriers in trade with the countries of the European Union.
The process of registration of cosmetic products will become radically new after the entry into force of the technical regulation.

It should be noted that there is a transition period until the entry into force of the Technical Regulation. This gives market operators time to prepare. The sale of cosmetics manufactured before 08/03/2026 will not be prohibited or restricted for the next 5 years.
If you are a manufacturer of cosmetics and are planning further development of your own production, you should approach this issue strategically now. The transition period in legislation is always a time of additional actions and new steps. In order not to waste time and avoid a number of unfortunate mistakes when registering cosmetic products under the new rules – contact MIASPHERA! We will provide full legal support and support at all stages of bringing cosmetic products to the market of Ukraine! MIASPHERA specialists will gladly share their successful experience!
What Miasphera can do for a cosmetics manufacturer:
We are always one step ahead and work according to new standards!
Would you like to receive a consultation? Leave an application using the link.
If you are a manufacturer or exporter of cosmetic products and have a desire to conquer the market of the European Union countries, you must do the registration procedure for your cosmetic products.
The procedure goes through the assessment of the conformity of cosmetic products in accordance with EU regulations and directives. If say briefly: your product must meet EU standards for this type of product.
Requirements for cosmetic products on the EU market are regulated by Regulation №1223/2009.
The regulation prohibits cosmetic products whose ingredients or final composition have been tested on animals from entering the EU market. Carcinogenic, mutagenic or toxic substances for reproductive health are also prohibited for use.
Conformity assessment consists of two main procedures: drawing up a Product Safety Report (CPSR) and notification. It sounds scary, but in this article we will explain this confusing issue.
Cosmetic products include a wide range of products. Direct contact with the human body is one common feature. It is this feature that is the reason for careful study of the physicochemical parameters of any cosmetic product.
A Safety Report (CPSR) allows you to make sure that the product under investigation is safe for use. It contains important data on the results of research on the composition and individual components. The procedure includes the creation of an information file, where each tool is marked with the appropriate marking and conditions of production compliance with EU legislation.
Notification is the process of entering cosmetic products into the pan-European database (CPNP). This process based on the basis of a product safety report.
So, we’re having dealt with the definition of the main terms! Now we can form the stages on the way to a successful procedure for evaluating the conformity of cosmetic products:

Successful completion of the first stage allows you to create a road map of the entire process of product registration and the final list of necessary documents for the creation of a dossier – PIF file.
In the case of registration of cosmetic products on the territory of the European Union countries, it is necessary to take care of the presence of an authorized representative.
This responsible person must register on the territory of the EU country, has full information about the products, is legally responsible for compliance with the requirements of cosmetics regulations and, if necessary, provides all necessary information at the request of supervisory authorities for control and monitoring of activities.
According to the Cosmetic Regulation (EU) №1223/2009, the authorized representative is legally responsible for a cosmetic product of a non-European manufacturer on the EU market.
All cosmetic products entering the EU market must have the following package of documents:

The Safety Data Sheet (MSDS) of cosmetic products is developed based on the data of the manufacturer and the authorized person. It contains more detailed information about: composition, possible risks of harm to humans, rules of conduct in case of improper use, storage and disposal conditions. This document is important not only for export operations, but also for the end user to ensure the correct and safe use of the product.
Registration may be refused if the cosmetics do not comply with the established safety standards of a certain country or if an incomplete package of documents is submitted.
Registration of medical devices in Ukraine, as well as in other countries of the world, implies their legalization and is necessary for the manufacturer to put medical devices on the market.
Since 2015, Ukraine has changed the procedure for state registration of medical devices and introduced a procedure for assessing compliance with the requirements of technical regulations for medical devices, including in vitro diagnostics and active implantable medical devices, as provided for by the Law of Ukraine “On Technical Regulations and Conformity Assessment”.
The most important thing to note right away is that quarantine measures aimed at preventing the spread of COVID-19 have not been canceled in Ukraine, and unfortunately, due to the military aggression (war) of the Russian Federation against Ukraine, the procedure for assessing compliance with the requirements of technical regulations on medical devices with the involvement of the designated body is temporarily carried out remotely. Remote audit of a medical device manufacturer has the following advantages: speed of audit and cost of such audit. The manufacturer of medical devices does not need to pay for the flight of auditors and an interpreter from Ukraine to the country of manufacture of medical devices, pay for hotel accommodation, per diem expenses, etc.
Therefore, the Technical Regulations approved in Ukraine comply with the following European Directives: Council Directive 93/42/EEC concerning medical devices, Directive 98/79/EC on in vitro diagnostic medical devices, Council Directive 90/385/EEC concerning active implantable medical devices.
Accordingly, the registration of medical devices in Ukraine is carried out in accordance with the procedure established by the Technical Regulations, which depends on the type and risk class of the medical device.
1. without involvement of the designated conformity assessment body, by means of the manufacturer’s internal control of compliance with the requirements of the Technical Regulations;
2. with the involvement of the designated conformity assessment body, by assessing the manufacturer’s quality management system.
The first method is the simplest and provides for the completion of internal control by drawing up and signing a declaration of conformity of medical devices with the requirements of technical regulations under the full responsibility of the manufacturer. Based on the results of internal control, the authorized representative retains the documents received from the manufacturer for medical devices – the technical file, developed labels, instructions – not only for the period of sale of medical devices, but also for up to 15 years after the sale of the last product. After executing the declaration and receiving the documents for medical devices, the authorized representative must make an entry in the Register of Persons Responsible for Placing Medical Devices on the Market. From the date of making such an entry, the manufacturer and/or its authorized representative are entitled to import medical devices into Ukraine at a reduced rate of value added tax and sell such devices on the Ukrainian market.
In general, the second method is a more complicated and time-consuming procedure, which is divided into several stages: application to the conformity assessment body with the relevant application form, which must be accompanied by questionnaire forms and lists of medical devices; examination of documents for medical devices – technical file; audit of the manufacturer’s quality management system. Based on the results of the audit, the designated conformity assessment body issues a certificate of conformity. Upon receipt of the certificate of conformity, the manufacturer and/or an authorized representative shall execute and sign a declaration of conformity of medical devices with the requirements of technical regulations under the full responsibility of the manufacturer.
At present, there are all prerequisites for the registration of medical devices in Ukraine to be profitable for the manufacturer, costing several times less and being faster. Therefore, it is time for global manufacturers of medical devices to discover the Ukrainian medical market and take a worthy place in it.
The registration of medical devices in Ukraine, which are urgently needed in all medical areas due to the restoration of critical medical infrastructure, will give a global manufacturer an advantage in developing the Ukrainian medical market.