The first mentions of the cultivation of cannabis by mankind date back to 4000 BC. This plant has been used for thousands of years as a medicine, as a source of food, fiber, oil and paper.
The history of mankind’s interrelations with cannabis has always been influenced by various cultural, social and local trends. Cannabis was used to treat various diseases in ancient China and India. Cannabis was used in the treatment of constipation, malaria, rheumatic pains and women’s ailments. The one spread to Africa and Arab countries in the Middle Ages, where it was used mostly in medicine, as well as in cultural and religious rituals. Marijuana was first used as a pain reliever in Ancient Greece. Cannabis spread to Europe and America in the 15th century, where its use became especially popular among doctors and artists.
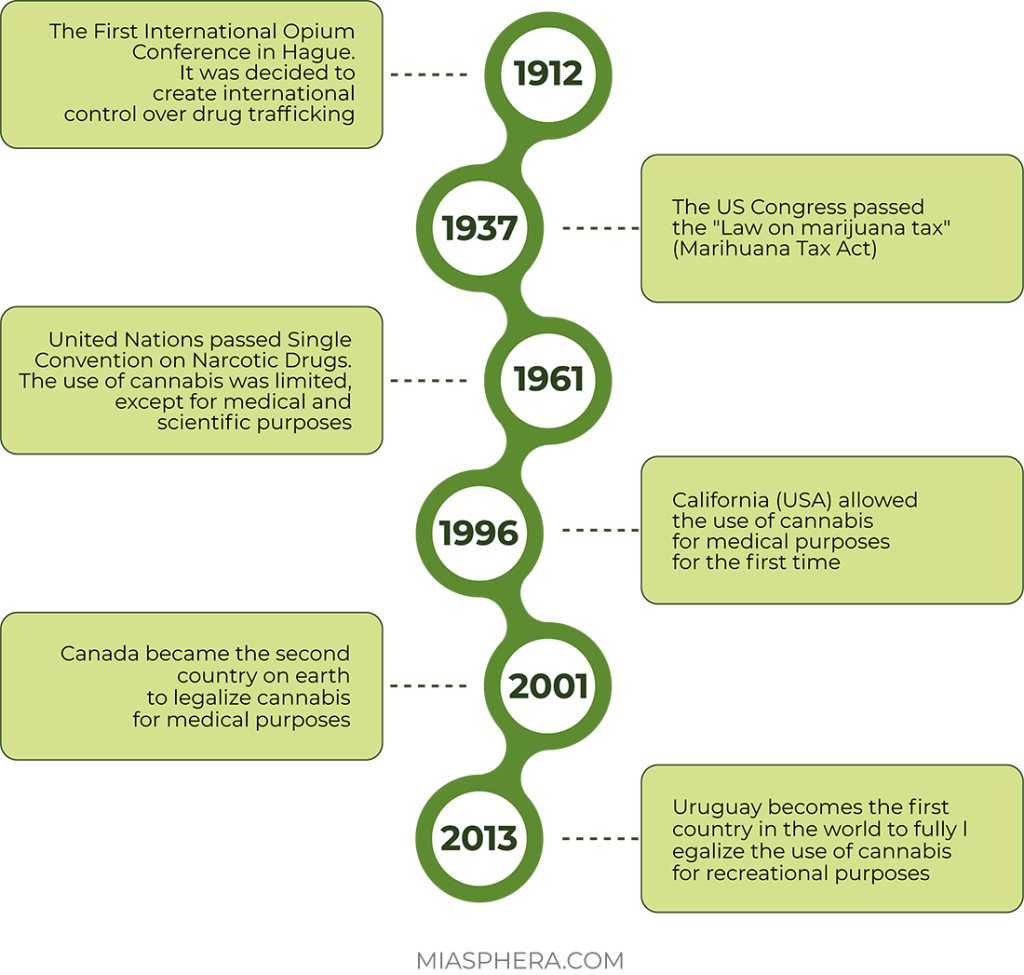
At the beginning of the 20th century, there was a rapid growth in the consumption of cannabis, including due to its recreational properties. At the same time, despite its great popularity, cannabis began to be banned en masse. It all started with the international opium convention signed in 1912. Initial control of the circulation of hard drugs and cannabis was introduced between the participating countries: Germany, Prussia, the Russian Empire, Great Britain, the Netherlands and many others. Marijuana was allowed to be produced and used only for medical purposes.
During the 1930s, an anti-marijuana campaign arose in the United States. The cannabis tax law was introduced in 1937.
At the same time, the popularity of hemp continued to grow in industry, for example for the production of ropes, shoes and clothing. In 1942, Henry Ford creates an experimental body made of hemp, which turned out to be 10 times stronger than steel of the same thickness. Hemp biodiesel was supposed to be the fuel for the car.
In the 1960s and 1970s, marijuana became especially popular among the counterculture youth, who became its main consumers. It becomes a kind of symbol of the hippie movement, as well as musical and artistic self-expression.
During this period, cannabis becomes the object of prohibition in many countries due to the danger of abuse and its psychotropic properties. The world history of marijuana takes a new turn in March 1961. The UN adopted the Single Convention on Narcotic Drugs. According to the cannabis convention, cannabis resin, cannabis extracts and tinctures were included in the list of narcotic drugs. The signatory countries were obliged to strengthen control over the cultivation of the plant, and to completely stop its use for purposes other than medical and scientific. Cultivation of industrial varieties of cannabis did not fall under the ban.
Many countries began the process of legalizing cannabis for medical and recreational purposes already at the end of the 20th century. It was an example of breaking traditional stereotypes.
Legal access to herbal cannabis and its use for medical purposes under the supervision of a doctor was first allowed in the state of California (USA) in 1996. In 2001, Canada became the second country in the world to legalize the use of cannabis for medical purposes. Since October 17, 2018, the use of cannabis for medical purposes has been allowed.
On December 10, 2013, the Uruguayan Senate passed a law on the complete decriminalization of the cultivation, sale, purchase and use of marijuana. Uruguay became the first country in the world to fully legalize cannabis.
In December 2020, the UN Commission on Narcotic Drugs removed medical cannabis and all its derivatives from the list of critically dangerous drugs, but about 25 countries still voted against this initiative.
Currently, the world market of cannabis, including for medical purposes, is in the process of rapid development. However, this development is not uniform due to different legal environments and mixed public attitudes.
In contrast to the legalization of recreational use of cannabis, more and more countries allow its medical use every year. In the last 5 years, about 20 countries in Europe (Greece, Estonia, Ireland, Luxembourg, Malta, Macedonia, Germany, Norway, Poland, Portugal, Croatia), North and South America (Argentina, Colombia, Mexico, Peru, Chile) and other countries (Australia, Philippines, Jamaica) have legalized cannabis and cannabinoids for medical purposes.
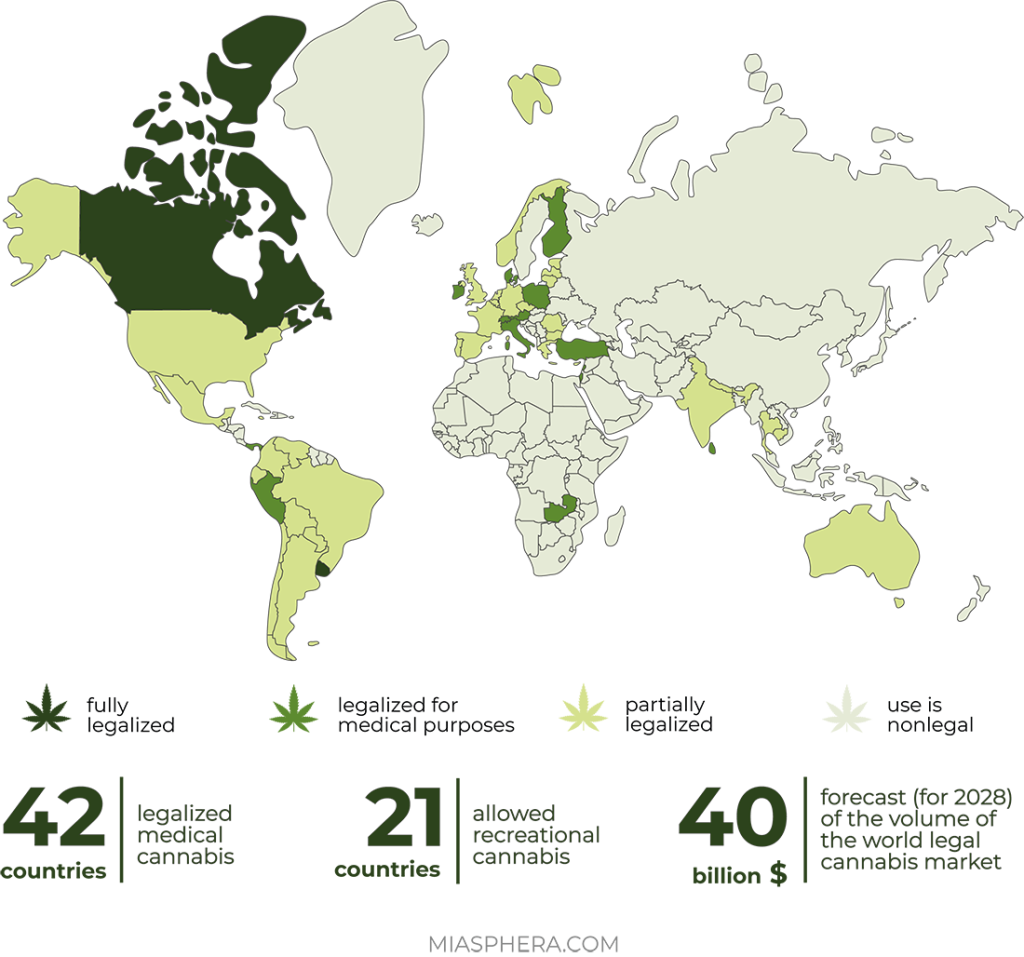
Some countries, such as Canada and the US, have adopted fully regulated frameworks that allow the cultivation, consumption and retail distribution of various cannabis-based products. So in South Africa, it is legally allowed to grow hemp, but not legalized retail sales.
In general, despite the slow changes, the world is witnessing the liberalization of the legal framework of international documents regarding the circulation of cannabis compounds and cannabinoids for medical purposes and the decriminalization of their use. Such drugs and preparations are gradually becoming available to patients in different countries.
The large number of restrictions on the production and use of cannabis in different countries often lead to misunderstandings and confusion in the concepts themselves. Not everyone realizes why there are three different names – cannabis, marijuana and hemp and what exactly is the difference between them. And those who do not know about the existence of industrial varieties are hostile to all cannabis in general.
So, cannabis, hemp or marijuana – which is correctly?
Answer: all names are correct! But from the point of view of botany, medicine and law are completely different things. There are different subspecies and products made from the same hemp. Let’s figure out what hemp is and why there’s been so much talk about it.
The plant of the genus Cannabis or Hemp is an agricultural crop characterized by jagged leaves and a characteristic smell. Botanists divide these plants by structure and classify them based on morphology, physiology, and origin. The most famous subspecies are Sativa and Indica or their hybrids.
Sativa is a tall plant with thin leaves that is traditionally grown in Latin America, North Africa, and Southeast Asia. The subspecies has an elevated level of tetrahydrocannabinol (THC).
Indica is a low-growing subspecies with a high level of cannabidiol (CBD) and is grown mainly in the mountainous regions of India and Pakistan.
There is also a wild subspecies – Ruderalis. It is less common, but on its basis all cultivated varieties of technical hemp with almost zero content of tetrahydrocannabinol (THC) were bred. It is known for its feature of autoflowering, depending on age, not light cycles. Due to its low THC content, it is not used for recreational or medical purposes.
In general, cannabis is the Latin name of the plant, which also refers to medical hemp varieties and hemp-based products for therapeutic purposes. Traditionally, medical cannabis has a balanced CBD content and low THC levels.
Marijuana is a smoking mixture of dried female flowers of sativa and indica. Marijuana has a higher percentage of psychoactive substances (THC more than 0.3%) compared to hemp and has psychotropic properties (a mild drug).
Why are there three names?
In connection with the legalization of medical cannabis, it became necessary to name different subspecies of hemp and products based on them. Thus, manufacturers distanced those who needed to use these plants specifically for medicinal purposes from users for recreational consumption.
How to determine the legal status of the cannabis plant?
These names should be clearly distinguished. So, knowing the properties, we can summarize that:
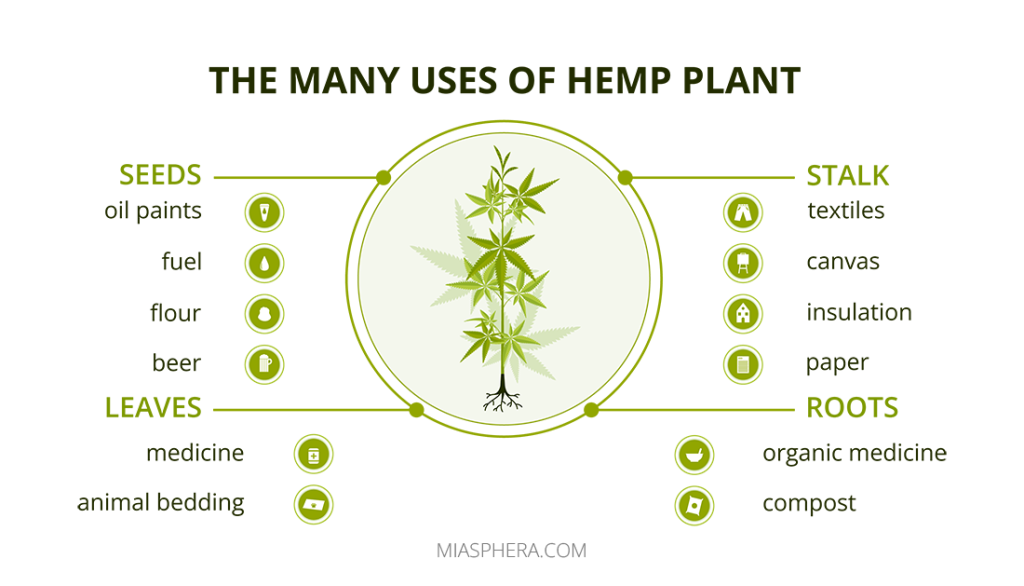
Hemp is an agricultural crop that includes all varieties of Cannabis Ruderalis. Hemp is a source of ecological textiles and paper, widely used for industrial purposes.
Cannabis is the designation of specialized varieties bred for therapeutic purposes, regardless of their narcotic properties.
Marijuana is a smoking mixture of dried female flowers of sativa and indica, which has the properties of a light narcotic.
Medical Marijuana or Medical Cannabis – This term is used specifically for the Sativa derivatives of cannabis that are used to relieve the symptoms caused by certain diseases.
What is the difference between medical and recreational marijuana?
The main difference is in the THC content. It is incorrect to equate medical marijuana with recreational marijuana, since its purpose is not to bring pleasure, but to relieve pain symptoms. And therefore, medical cannabis is specially bred varieties with a changed THC content.
Currently, many varieties of cannabis Sativa plants have been bred specifically for medical use.
The main active substance is cannabidiol (CBD). The results of scientific research show that herbal preparations with CBD effectively block various types of pain, increase appetite, help overcome depressive states, and prevent epileptic attacks. This is an important remedy for people suffering from constant pain, oncology and AIDS.
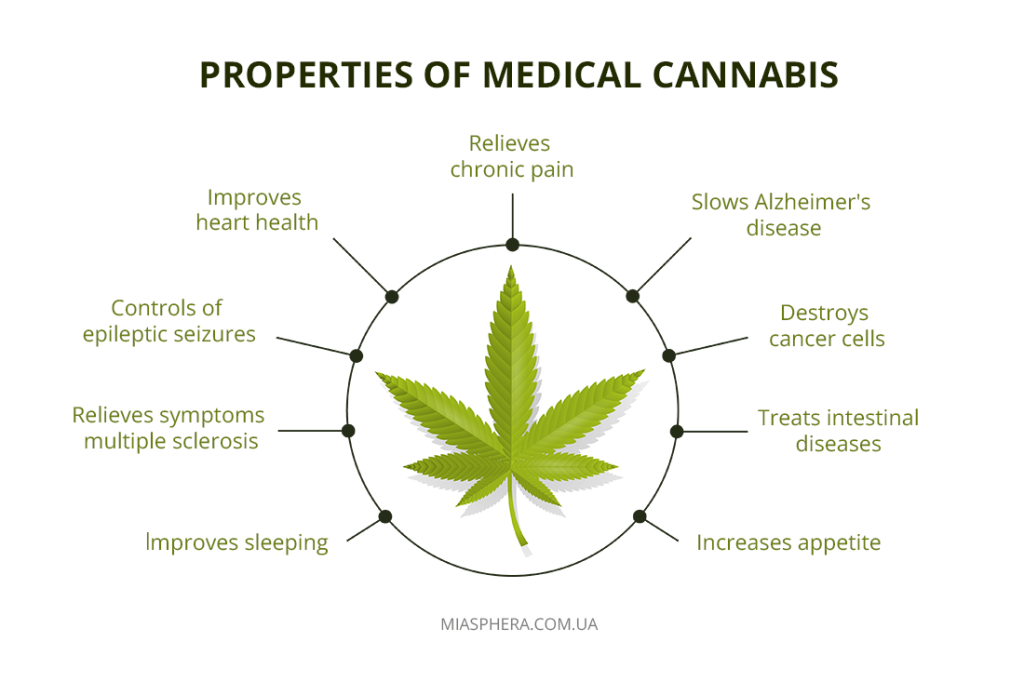
Medical cannabis has shown potential in alleviating symptoms associated with neurological diseases such as epilepsy, multiple sclerosis, Parkinson’s disease, Alzheimer’s disease, and neuropathic pain. But it is important to understand that cannabis is not a panacea and such treatment may not be useful for everyone.
Currently, numerous studies are being conducted around the world on the effect of medical cannabis in the treatment of particularly complex diseases.
But at the same time, the ambiguous legal status of different chemotypes makes it difficult to study the properties of cannabis. A mandatory condition for conducting research is a special license, obtaining which is a rather bureaucratic procedure.
We hope that science will stubbornly move forward in this matter, discovering new useful ways of using hemp and its derivatives in medicine, chemical industry, industry and agriculture.
WARNING! The article is posted for educational purposes and does not promote the use of drugs. We hope our publication brought a scientific understanding of the topic and helped to understand the differences in terms.
Today, every global medical device manufacturer is undoubtedly facing a difficult question: “Should I import medical devices to Ukraine?” And the answer is very simple: “Yes, of course”.
For many global medical device manufacturers that were present on the Ukrainian market before the war, the current permits for medical devices are now a stellar time, a time when Ukraine is gradually rebuilding and restoring its damaged medical infrastructure.
To support the positive answer to the above question regarding the import of medical devices to Ukraine, we provide statistical data:
The state budget of Ukraine for 2022 allocates UAH 195.9 billion for healthcare, and with additional funding due to the full-scale war – UAH 232.8 billion. In 2023, the budget allocates UAH 175 billion for healthcare, including UAH 10.3 billion for centralized procurement of medical products in 31 areas in 2023.
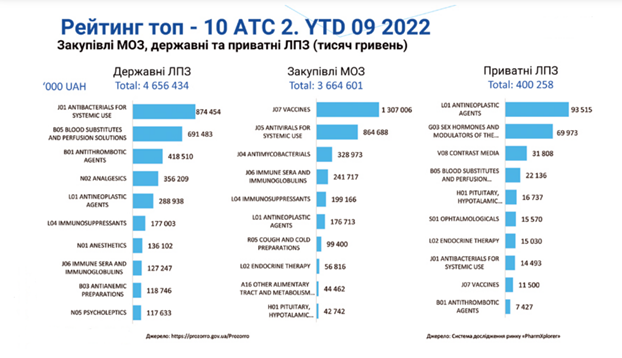
However, Ukraine is on a course to standardize with Europe, and while restoring and rebuilding the lost medical infrastructure, it aims to introduce the latest technologies and products, including medical devices.
Therefore, it is time for global manufacturers with the latest and most advanced medical technologies and products to discover the Ukrainian market and start importing medical devices to Ukraine.
An Authorized Representative appointed in Ukraine will help a global manufacturer of medical devices to overcome all the difficulties related to the import of medical devices to Ukraine. Since any import of medical devices to Ukraine for manufacturers of medical devices that are not residents of Ukraine is not possible without the appointment of an Authorized Representative in Ukraine.
It is the Authorized Representative, who may be a trade participant or solely the holder of registration documents for a medical device, who takes all the necessary actions to ensure that the import of medical devices to Ukraine is successful.
Importation requires a number of mandatory actions and additional actions at the choice of the medical device manufacturer. It is mandatory to conclude an agreement with a company from Ukraine on the provision of services of an Authorized Representative.
Having confirmation of his/her authority, the Authorized Representative examines the technical documentation for the medical device in order to establish the necessary procedure for assessing the conformity of the medical device and subsequently initiates its implementation. In other words, import of medical devices to Ukraine that are class I medical devices, non-sterile, without measurement function and medical devices for in vitro diagnostics, and not intended for self-monitoring is possible upon registration of the manufacturer and the Authorized Representative in the Register of the State Service of Ukraine on Medicines and Drugs Control. Instead, medical devices of a higher risk class are subject to the manufacturer’s audit procedure with the involvement of the designated medical device conformity assessment body.
Having such documents as a declaration of conformity of medical devices and/or a certificate of conformity assessment for a medical device, the most difficult stages before importation are completed.
Ukrainian legislation allows importing medical devices into Ukraine subject to confirmation of their compliance with the Technical Regulations (as evidenced by the aforementioned declaration and/or certificate), the label for the medical device developed in Ukrainian, and the instruction for use of the medical device.
Due to the fact that in Ukraine, as in other countries, the circulation of medical devices is regulated, a number of requirements are set for the said labels and instructions for use of the medical device in terms of their form and content. This complex task is undertaken by the Authorized Representative, and the medical device manufacturer will only need to provide technical files for the medical device, which will be processed by the Authorized Representative to develop the correct label and instruction for the medical device.
The above-mentioned documents are subject to inspection at the customs during the regulated procedures for customs clearance of medical devices in Ukraine, which are determined depending on the medical device, according to the UKTZED code for the product.
From this stage, the manufacturer of medical devices can carry out the production of such devices in order to import medical devices to Ukraine. And the benefit of the Authorized Representative at the following stages is no less important, since the Authorized Representative can search for a logistics partner for the import of goods, advise a reliable dealer, and take actions aimed at preventing gray imports of medical devices by the manufacturer in the Ukrainian market. The above actions are additional for the medical device manufacturer, but no less important for the successful import and sale of medical devices in the Ukrainian market.
Importing medical devices to Ukraine is simple, not expensive and predictably successful if you entrust this business to a reliable partner – an Authorized Representative.
Import medical devices to Ukraine – join the rebuilding and restoration of Ukraine, for peace, justice and democratic values.