Registration of cosmetics in Ukraine is one of the popular queries in search engines. This is not surprising, because the trend of the last decade is the growth of the cosmetic market. This is indicated by a stable increase in the volume of production and sale of cosmetic products. Cosmetics have long become not just a product for care, but also a necessary element of a person’s daily use.
The Ukrainian market of cosmetic products is no exception. Therefore, the issue of the appearance of new products, increasing the number of manufacturers and technical regulation of the circulation of cosmetics is an urgent issue for Ukrainian legislation.
How to legally register cosmetic products in Ukraine?
Is the certification of cosmetics mandatory, and when does the new Technical Regulation come into effect? Vitaliy Kovbasa, the chief lawyer of MIASPHERA company, answers these and other questions.
We will provide some theory and understanding of the term “cosmetic products” from the Ukrainian legislation.
Cosmetic products in Ukraine are regulated in accordance with the Law On Cosmetic Products dated 04/05/2012 No. 4616-VI.
Any substance or mixture of substances can be considered a cosmetic product. They are intended for use on the external parts of the human body in order to clean, aromatize, restore, protect and correct the smell.
Every foreign manufacturer or importer that brings cosmetic products to the market of Ukraine undergoes a simplified procedure for declaring compliance. Cosmetics from the EU already meet the standards that will soon be implemented in our country. It is enough for the importer to provide a certificate from the manufacturer and a safety data sheet for the products.
The national producer has to do this procedure by obtaining a conclusion from a sanitary-epidemiological examination of production and products.
Registration of cosmetic products in Ukraine for a national manufacturer is carried out through the voluntary receipt of a sanitary-hygienic expert opinion, which confirms the safety of products for human health and gives the right to sell them. This procedure is valid until the Technical Regulation is fully implemented.
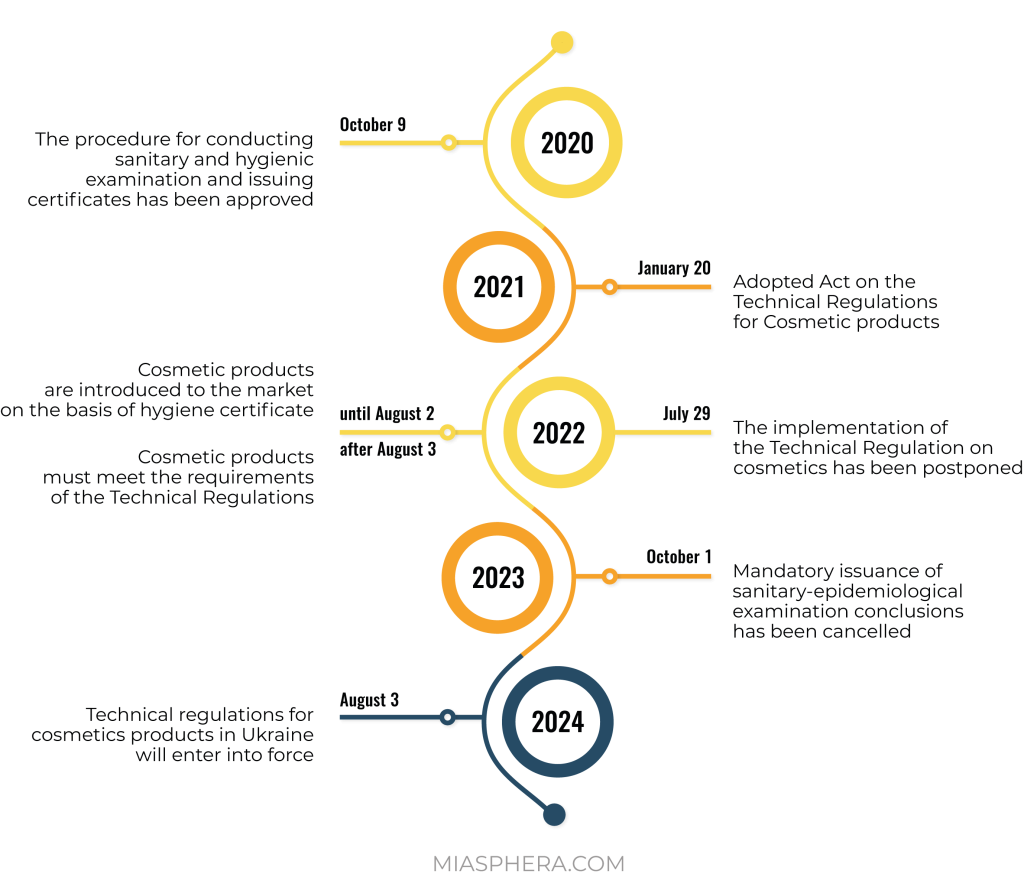
The process of regulation of cosmetic products in Ukraine is actively integrated with the legislation of the European Union. On the basis of EU Regulation No. 1223/2009, a technical regulation for cosmetic products was developed for Ukraine. This normative act was postponed several times, but was still adopted by the Cabinet of Ministers on January 20, 2021.
In connection with the hostilities, the Ministry of Health proposed postponing the entry into force of the Technical Regulation until August 3, 2024, and introduced a transition period for participants in the cosmetic products market until August 3, 2026.
The main purpose of introducing the Technical Regulation is to establish new standards for cosmetic products on the market of Ukraine, to define the rights and obligations of market participants regarding the introduction of cosmetic products into circulation, as well as to remove administrative barriers in trade with the countries of the European Union.
The process of registration of cosmetic products will become radically new after the entry into force of the technical regulation.

It should be noted that there is a transition period until the entry into force of the Technical Regulation. This gives market operators time to prepare. The sale of cosmetics manufactured before 08/03/2026 will not be prohibited or restricted for the next 5 years.
If you are a manufacturer of cosmetics and are planning further development of your own production, you should approach this issue strategically now. The transition period in legislation is always a time of additional actions and new steps. In order not to waste time and avoid a number of unfortunate mistakes when registering cosmetic products under the new rules – contact MIASPHERA! We will provide full legal support and support at all stages of bringing cosmetic products to the market of Ukraine! MIASPHERA specialists will gladly share their successful experience!
What Miasphera can do for a cosmetics manufacturer:
We are always one step ahead and work according to new standards!
Would you like to receive a consultation? Leave an application using the link.
If you are a manufacturer or exporter of cosmetic products and have a desire to conquer the market of the European Union countries, you must do the registration procedure for your cosmetic products.
The procedure goes through the assessment of the conformity of cosmetic products in accordance with EU regulations and directives. If say briefly: your product must meet EU standards for this type of product.
Requirements for cosmetic products on the EU market are regulated by Regulation №1223/2009.
The regulation prohibits cosmetic products whose ingredients or final composition have been tested on animals from entering the EU market. Carcinogenic, mutagenic or toxic substances for reproductive health are also prohibited for use.
Conformity assessment consists of two main procedures: drawing up a Product Safety Report (CPSR) and notification. It sounds scary, but in this article we will explain this confusing issue.
Cosmetic products include a wide range of products. Direct contact with the human body is one common feature. It is this feature that is the reason for careful study of the physicochemical parameters of any cosmetic product.
A Safety Report (CPSR) allows you to make sure that the product under investigation is safe for use. It contains important data on the results of research on the composition and individual components. The procedure includes the creation of an information file, where each tool is marked with the appropriate marking and conditions of production compliance with EU legislation.
Notification is the process of entering cosmetic products into the pan-European database (CPNP). This process based on the basis of a product safety report.
So, we’re having dealt with the definition of the main terms! Now we can form the stages on the way to a successful procedure for evaluating the conformity of cosmetic products:

Successful completion of the first stage allows you to create a road map of the entire process of product registration and the final list of necessary documents for the creation of a dossier – PIF file.
In the case of registration of cosmetic products on the territory of the European Union countries, it is necessary to take care of the presence of an authorized representative.
This responsible person must register on the territory of the EU country, has full information about the products, is legally responsible for compliance with the requirements of cosmetics regulations and, if necessary, provides all necessary information at the request of supervisory authorities for control and monitoring of activities.
According to the Cosmetic Regulation (EU) №1223/2009, the authorized representative is legally responsible for a cosmetic product of a non-European manufacturer on the EU market.
All cosmetic products entering the EU market must have the following package of documents:

The Safety Data Sheet (MSDS) of cosmetic products is developed based on the data of the manufacturer and the authorized person. It contains more detailed information about: composition, possible risks of harm to humans, rules of conduct in case of improper use, storage and disposal conditions. This document is important not only for export operations, but also for the end user to ensure the correct and safe use of the product.
Registration may be refused if the cosmetics do not comply with the established safety standards of a certain country or if an incomplete package of documents is submitted.
The large number of restrictions on the production and use of cannabis in different countries often lead to misunderstandings and confusion in the concepts themselves. Not everyone realizes why there are three different names – cannabis, marijuana and hemp and what exactly is the difference between them. And those who do not know about the existence of industrial varieties are hostile to all cannabis in general.
So, cannabis, hemp or marijuana – which is correctly?
Answer: all names are correct! But from the point of view of botany, medicine and law are completely different things. There are different subspecies and products made from the same hemp. Let’s figure out what hemp is and why there’s been so much talk about it.
The plant of the genus Cannabis or Hemp is an agricultural crop characterized by jagged leaves and a characteristic smell. Botanists divide these plants by structure and classify them based on morphology, physiology, and origin. The most famous subspecies are Sativa and Indica or their hybrids.
Sativa is a tall plant with thin leaves that is traditionally grown in Latin America, North Africa, and Southeast Asia. The subspecies has an elevated level of tetrahydrocannabinol (THC).
Indica is a low-growing subspecies with a high level of cannabidiol (CBD) and is grown mainly in the mountainous regions of India and Pakistan.
There is also a wild subspecies – Ruderalis. It is less common, but on its basis all cultivated varieties of technical hemp with almost zero content of tetrahydrocannabinol (THC) were bred. It is known for its feature of autoflowering, depending on age, not light cycles. Due to its low THC content, it is not used for recreational or medical purposes.
In general, cannabis is the Latin name of the plant, which also refers to medical hemp varieties and hemp-based products for therapeutic purposes. Traditionally, medical cannabis has a balanced CBD content and low THC levels.
Marijuana is a smoking mixture of dried female flowers of sativa and indica. Marijuana has a higher percentage of psychoactive substances (THC more than 0.3%) compared to hemp and has psychotropic properties (a mild drug).
Why are there three names?
In connection with the legalization of medical cannabis, it became necessary to name different subspecies of hemp and products based on them. Thus, manufacturers distanced those who needed to use these plants specifically for medicinal purposes from users for recreational consumption.
How to determine the legal status of the cannabis plant?
These names should be clearly distinguished. So, knowing the properties, we can summarize that:
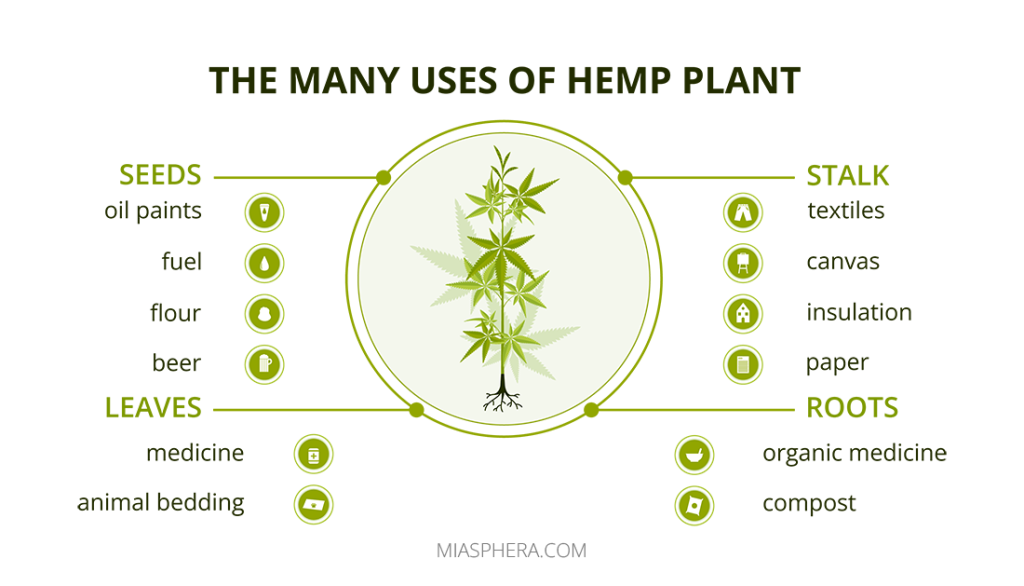
Hemp is an agricultural crop that includes all varieties of Cannabis Ruderalis. Hemp is a source of ecological textiles and paper, widely used for industrial purposes.
Cannabis is the designation of specialized varieties bred for therapeutic purposes, regardless of their narcotic properties.
Marijuana is a smoking mixture of dried female flowers of sativa and indica, which has the properties of a light narcotic.
Medical Marijuana or Medical Cannabis – This term is used specifically for the Sativa derivatives of cannabis that are used to relieve the symptoms caused by certain diseases.
What is the difference between medical and recreational marijuana?
The main difference is in the THC content. It is incorrect to equate medical marijuana with recreational marijuana, since its purpose is not to bring pleasure, but to relieve pain symptoms. And therefore, medical cannabis is specially bred varieties with a changed THC content.
Currently, many varieties of cannabis Sativa plants have been bred specifically for medical use.
The main active substance is cannabidiol (CBD). The results of scientific research show that herbal preparations with CBD effectively block various types of pain, increase appetite, help overcome depressive states, and prevent epileptic attacks. This is an important remedy for people suffering from constant pain, oncology and AIDS.
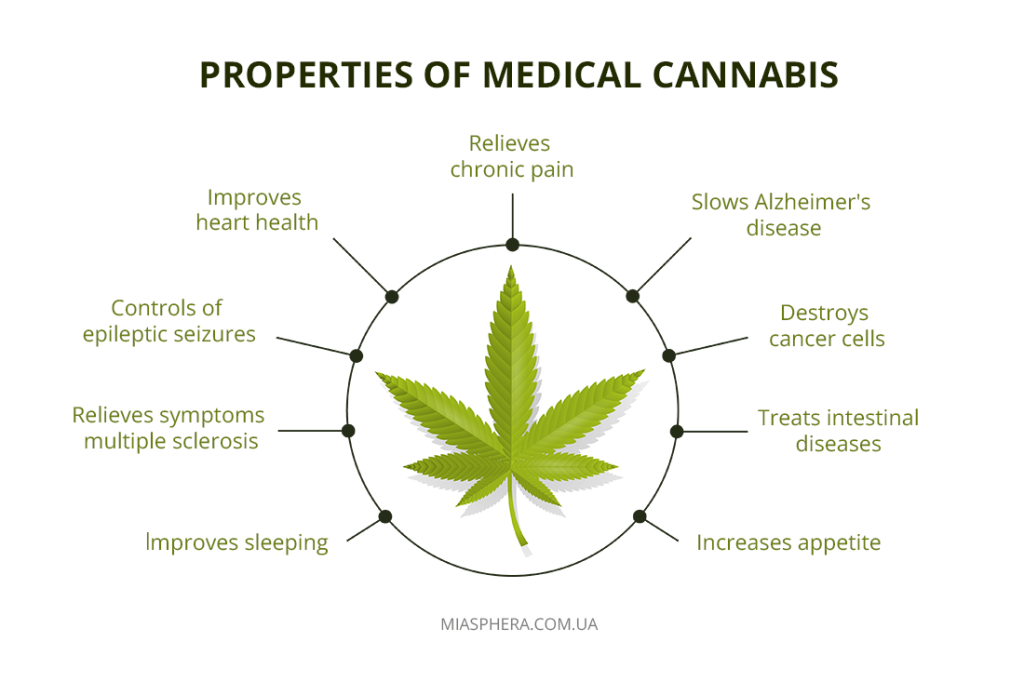
Medical cannabis has shown potential in alleviating symptoms associated with neurological diseases such as epilepsy, multiple sclerosis, Parkinson’s disease, Alzheimer’s disease, and neuropathic pain. But it is important to understand that cannabis is not a panacea and such treatment may not be useful for everyone.
Currently, numerous studies are being conducted around the world on the effect of medical cannabis in the treatment of particularly complex diseases.
But at the same time, the ambiguous legal status of different chemotypes makes it difficult to study the properties of cannabis. A mandatory condition for conducting research is a special license, obtaining which is a rather bureaucratic procedure.
We hope that science will stubbornly move forward in this matter, discovering new useful ways of using hemp and its derivatives in medicine, chemical industry, industry and agriculture.
WARNING! The article is posted for educational purposes and does not promote the use of drugs. We hope our publication brought a scientific understanding of the topic and helped to understand the differences in terms.
Today, every global medical device manufacturer is undoubtedly facing a difficult question: “Should I import medical devices to Ukraine?” And the answer is very simple: “Yes, of course”.
For many global medical device manufacturers that were present on the Ukrainian market before the war, the current permits for medical devices are now a stellar time, a time when Ukraine is gradually rebuilding and restoring its damaged medical infrastructure.
To support the positive answer to the above question regarding the import of medical devices to Ukraine, we provide statistical data:
The state budget of Ukraine for 2022 allocates UAH 195.9 billion for healthcare, and with additional funding due to the full-scale war – UAH 232.8 billion. In 2023, the budget allocates UAH 175 billion for healthcare, including UAH 10.3 billion for centralized procurement of medical products in 31 areas in 2023.
However, Ukraine is on a course to standardize with Europe, and while restoring and rebuilding the lost medical infrastructure, it aims to introduce the latest technologies and products, including medical devices.
Therefore, it is time for global manufacturers with the latest and most advanced medical technologies and products to discover the Ukrainian market and start importing medical devices to Ukraine.
An Authorized Representative appointed in Ukraine will help a global manufacturer of medical devices to overcome all the difficulties related to the import of medical devices to Ukraine. Since any import of medical devices to Ukraine for manufacturers of medical devices that are not residents of Ukraine is not possible without the appointment of an Authorized Representative in Ukraine.
It is the Authorized Representative, who may be a trade participant or solely the holder of registration documents for a medical device, who takes all the necessary actions to ensure that the import of medical devices to Ukraine is successful.
Importation requires a number of mandatory actions and additional actions at the choice of the medical device manufacturer. It is mandatory to conclude an agreement with a company from Ukraine on the provision of services of an Authorized Representative.
Having confirmation of his/her authority, the Authorized Representative examines the technical documentation for the medical device in order to establish the necessary procedure for assessing the conformity of the medical device and subsequently initiates its implementation. In other words, import of medical devices to Ukraine that are class I medical devices, non-sterile, without measurement function and medical devices for in vitro diagnostics, and not intended for self-monitoring is possible upon registration of the manufacturer and the Authorized Representative in the Register of the State Service of Ukraine on Medicines and Drugs Control. Instead, medical devices of a higher risk class are subject to the manufacturer’s audit procedure with the involvement of the designated medical device conformity assessment body.
Having such documents as a declaration of conformity of medical devices and/or a certificate of conformity assessment for a medical device, the most difficult stages before importation are completed.
Ukrainian legislation allows importing medical devices into Ukraine subject to confirmation of their compliance with the Technical Regulations (as evidenced by the aforementioned declaration and/or certificate), the label for the medical device developed in Ukrainian, and the instruction for use of the medical device.
Due to the fact that in Ukraine, as in other countries, the circulation of medical devices is regulated, a number of requirements are set for the said labels and instructions for use of the medical device in terms of their form and content. This complex task is undertaken by the Authorized Representative, and the medical device manufacturer will only need to provide technical files for the medical device, which will be processed by the Authorized Representative to develop the correct label and instruction for the medical device.
The above-mentioned documents are subject to inspection at the customs during the regulated procedures for customs clearance of medical devices in Ukraine, which are determined depending on the medical device, according to the UKTZED code for the product.
From this stage, the manufacturer of medical devices can carry out the production of such devices in order to import medical devices to Ukraine. And the benefit of the Authorized Representative at the following stages is no less important, since the Authorized Representative can search for a logistics partner for the import of goods, advise a reliable dealer, and take actions aimed at preventing gray imports of medical devices by the manufacturer in the Ukrainian market. The above actions are additional for the medical device manufacturer, but no less important for the successful import and sale of medical devices in the Ukrainian market.
Importing medical devices to Ukraine is simple, not expensive and predictably successful if you entrust this business to a reliable partner – an Authorized Representative.
Import medical devices to Ukraine – join the rebuilding and restoration of Ukraine, for peace, justice and democratic values.
Since the introduction of measures aimed at preventing the spread of the SARS-CoV-2 coronavirus infection, namely in March 2020, Ukraine has introduced the possibility of conducting a conformity assessment procedure for medical devices through a remote audit of the manufacturer. A certificate of conformity is a confirmation of completion of this procedure. In Ukraine, as in the rest of the world, the introduction into circulation and sale of medical devices is possible subject to confirmation of their compliance with the technical regulations on medical devices, i.e. obtaining a certificate of conformity and/or a declaration of conformity for medical devices.
Unfortunately, since February 24, 2022, the day of the military aggression of the Russian Federation against Ukraine, and in connection with the extension of quarantine restrictions due to the SARS-CoV-2 coronavirus infection, Ukraine has retained the possibility of conducting a conformity assessment procedure for medical devices through a remote audit of the manufacturer.
In order to obtain a certificate of conformity during martial law in Ukraine, a medical device manufacturer that is not a resident of Ukraine, through its Authorized Representative, applies to the designated conformity assessment body and initiates the process of conformity assessment of medical devices by signing an agreement.
At present, all designated conformity assessment bodies in Ukraine provide the possibility of passing the conformity assessment procedure for medical devices and, upon completion, issue a certificate of conformity during martial law in Ukraine.
In connection with the possibility of remote audit of the manufacturer under martial law, this procedure involves the evaluation of documents and records, which is carried out through the use of remote access.
Among the positive aspects of the remote audit of the manufacturer are the following
– high efficiency of the conformity assessment of medical devices;
– the ability to engage hard-to-reach personnel around the world and in several countries simultaneously;
– reduction of travel and communication costs;
– reduction of working time of the employees of the designated conformity assessment body.
In general, the reduction in the cost of the conformity assessment procedure by means of a manufacturer’s audit is a significant advantage. This advantage of the speed of remote audit and the lower cost of such a procedure compared to an on-site audit is compounded by the fact that the Ukrainian medical market currently requires a significant number of new medical devices, including new ones such as tactical medicine, rehabilitation and prosthetic devices.
According to a World Bank study, as of 2023, at least 978 medical facilities in Ukraine have been destroyed or damaged due to the war with Russia. Also, 650 ambulances and at least 596 pharmacies were damaged or destroyed. However, despite the ongoing war, the Ukrainian government is committed to restoring the lost medical infrastructure, and therefore the medical market needs more medical supplies than ever before, from bandages to medical equipment.
All of the above indicates that the medical market is open to global manufacturers of medical products of various kinds.
In particular, the market of laboratory tests, as exemplified by blood collection systems, is represented in Ukraine by 428 centers where blood is donated and stored, including 43 blood centers of regional and city significance; 309 transfusion departments; and 76 hospitals where donors are received. This medical infrastructure requires significant volumes of various consumables, as well as vacuum tubes for blood collection, needles for blood collection, containers for transportation and storage of blood, disinfectants, gloves and clothing for medical personnel, etc.
Therefore, the certificate of conformity during martial law in Ukraine continues to be issued by the designated conformity assessment bodies and is the result of the conformity assessment procedure for medical devices through a remote audit of the manufacturer.
Registration of medical devices in Ukraine, as well as in other countries of the world, implies their legalization and is necessary for the manufacturer to put medical devices on the market.
Since 2015, Ukraine has changed the procedure for state registration of medical devices and introduced a procedure for assessing compliance with the requirements of technical regulations for medical devices, including in vitro diagnostics and active implantable medical devices, as provided for by the Law of Ukraine “On Technical Regulations and Conformity Assessment”.
The most important thing to note right away is that quarantine measures aimed at preventing the spread of COVID-19 have not been canceled in Ukraine, and unfortunately, due to the military aggression (war) of the Russian Federation against Ukraine, the procedure for assessing compliance with the requirements of technical regulations on medical devices with the involvement of the designated body is temporarily carried out remotely. Remote audit of a medical device manufacturer has the following advantages: speed of audit and cost of such audit. The manufacturer of medical devices does not need to pay for the flight of auditors and an interpreter from Ukraine to the country of manufacture of medical devices, pay for hotel accommodation, per diem expenses, etc.
Therefore, the Technical Regulations approved in Ukraine comply with the following European Directives: Council Directive 93/42/EEC concerning medical devices, Directive 98/79/EC on in vitro diagnostic medical devices, Council Directive 90/385/EEC concerning active implantable medical devices.
Accordingly, the registration of medical devices in Ukraine is carried out in accordance with the procedure established by the Technical Regulations, which depends on the type and risk class of the medical device.
1. without involvement of the designated conformity assessment body, by means of the manufacturer’s internal control of compliance with the requirements of the Technical Regulations;
2. with the involvement of the designated conformity assessment body, by assessing the manufacturer’s quality management system.
The first method is the simplest and provides for the completion of internal control by drawing up and signing a declaration of conformity of medical devices with the requirements of technical regulations under the full responsibility of the manufacturer. Based on the results of internal control, the authorized representative retains the documents received from the manufacturer for medical devices – the technical file, developed labels, instructions – not only for the period of sale of medical devices, but also for up to 15 years after the sale of the last product. After executing the declaration and receiving the documents for medical devices, the authorized representative must make an entry in the Register of Persons Responsible for Placing Medical Devices on the Market. From the date of making such an entry, the manufacturer and/or its authorized representative are entitled to import medical devices into Ukraine at a reduced rate of value added tax and sell such devices on the Ukrainian market.
In general, the second method is a more complicated and time-consuming procedure, which is divided into several stages: application to the conformity assessment body with the relevant application form, which must be accompanied by questionnaire forms and lists of medical devices; examination of documents for medical devices – technical file; audit of the manufacturer’s quality management system. Based on the results of the audit, the designated conformity assessment body issues a certificate of conformity. Upon receipt of the certificate of conformity, the manufacturer and/or an authorized representative shall execute and sign a declaration of conformity of medical devices with the requirements of technical regulations under the full responsibility of the manufacturer.
At present, there are all prerequisites for the registration of medical devices in Ukraine to be profitable for the manufacturer, costing several times less and being faster. Therefore, it is time for global manufacturers of medical devices to discover the Ukrainian medical market and take a worthy place in it.
The registration of medical devices in Ukraine, which are urgently needed in all medical areas due to the restoration of critical medical infrastructure, will give a global manufacturer an advantage in developing the Ukrainian medical market.