The use of cannabis in the world for any purpose has always been the subject of intense attention among medical professionals and public opinion. And our country is no exception! The stages of legalization of medical cannabis in Ukraine are still going through a thorny path of legislative vicissitudes and changes.
But in the last year, the issue of the legal status of cannabis and its legalization in Ukraine has made significant progress.
An important event took place on February 13, 2023. The President of Ukraine signed Law №7457 on regulating the circulation of medical cannabis. The law will enter into force six months after its promulgation.
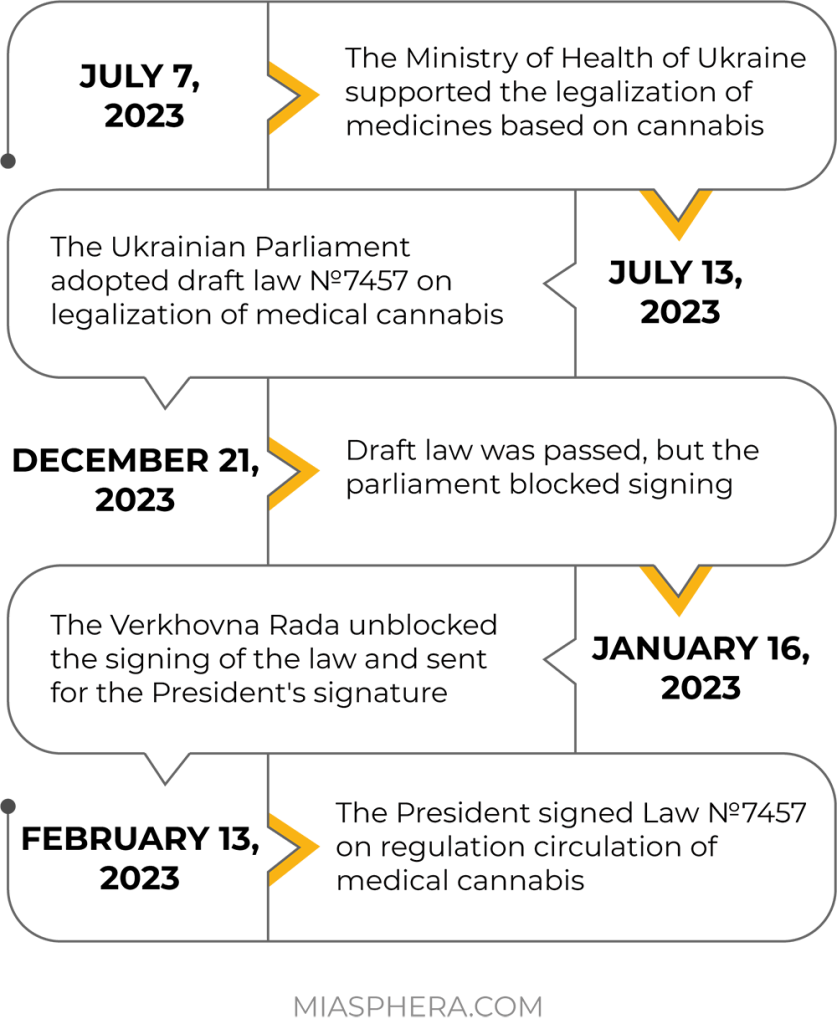
The main goal of the law is to increase access to medical cannabis preparations for the treatment of a number of diseases. According to the Ministry of Health, more than 2 million Ukrainians currently need cannabis-based drugs. The adopted law declares that the circulation of varieties of plants of the genus hemp (Cannabis) for medical purposes, products of their processing and medicines produced from them, is allowed only for the purpose of their use in medical, scientific and scientific and technical practice.
Distribution and consumption of cannabis for recreational purposes among the public will be prohibited. Therefore, a potentially important new tool can be considered the introduction of a traceability system for the circulation of varieties of hemp plants.
The issue of cannabis legalization in Ukraine opens up a wide space for discussion of new business prospects. This can be a significant stimulus for the development of new sectors of the economy, not only in the field of the production of medical and cosmetic preparations based on Cannabis plants, but also the industrial cultivation and processing of hemp. Further scientific research into the properties of existing varieties of hemp and their subspecies is also of interest.
In one case or another, the adoption of the law does not mean that cannabis plants and drugs based on them will be widely available to Ukraine already this year. We can safely predict that some time after the adoption of the law, the electronic registers defined by the draft law will be formed, algorithms for controlling the circulation of plants, conditions for granting quotas, etc. will be prescribed.

Vitaliy Kovbasa, a leading lawyer at the Miasphera consulting company, talks about the prospects and conditions for the legalization of cannabis-related activities in Ukraine.
“Rejecting skepticism and assessing the situation objectively, we can state this process will not be clearly defined in the nearest time. Those who wish to pursue cannabis-related activities in the future need to worry about the legal side now. The most important thing before starting such activities is to ensure the availability of appropriate licenses and pass the appropriate certification procedures,” Vitaliy comments.
What should be taken into account?
Licensing, namely obtaining a license for:
Certification, namely:
Therefore, all types of activities related to the circulation of hemp plants are allowed only if the subjects of such activities have the appropriate licenses and undergo the appropriate certification procedures.
Disclaimer: This article doesn’t promote the use of drugs or any other illegal substances. The text is a description of the history and scientific studies of the influence of cannabis on society, man, and his physical and mental health.
Since the introduction of measures aimed at preventing the spread of the SARS-CoV-2 coronavirus infection, namely in March 2020, Ukraine has introduced the possibility of conducting a conformity assessment procedure for medical devices through a remote audit of the manufacturer. A certificate of conformity is a confirmation of completion of this procedure. In Ukraine, as in the rest of the world, the introduction into circulation and sale of medical devices is possible subject to confirmation of their compliance with the technical regulations on medical devices, i.e. obtaining a certificate of conformity and/or a declaration of conformity for medical devices.
Unfortunately, since February 24, 2022, the day of the military aggression of the Russian Federation against Ukraine, and in connection with the extension of quarantine restrictions due to the SARS-CoV-2 coronavirus infection, Ukraine has retained the possibility of conducting a conformity assessment procedure for medical devices through a remote audit of the manufacturer.
In order to obtain a certificate of conformity during martial law in Ukraine, a medical device manufacturer that is not a resident of Ukraine, through its Authorized Representative, applies to the designated conformity assessment body and initiates the process of conformity assessment of medical devices by signing an agreement.
At present, all designated conformity assessment bodies in Ukraine provide the possibility of passing the conformity assessment procedure for medical devices and, upon completion, issue a certificate of conformity during martial law in Ukraine.
In connection with the possibility of remote audit of the manufacturer under martial law, this procedure involves the evaluation of documents and records, which is carried out through the use of remote access.
Among the positive aspects of the remote audit of the manufacturer are the following
– high efficiency of the conformity assessment of medical devices;
– the ability to engage hard-to-reach personnel around the world and in several countries simultaneously;
– reduction of travel and communication costs;
– reduction of working time of the employees of the designated conformity assessment body.
In general, the reduction in the cost of the conformity assessment procedure by means of a manufacturer’s audit is a significant advantage. This advantage of the speed of remote audit and the lower cost of such a procedure compared to an on-site audit is compounded by the fact that the Ukrainian medical market currently requires a significant number of new medical devices, including new ones such as tactical medicine, rehabilitation and prosthetic devices.
According to a World Bank study, as of 2023, at least 978 medical facilities in Ukraine have been destroyed or damaged due to the war with Russia. Also, 650 ambulances and at least 596 pharmacies were damaged or destroyed. However, despite the ongoing war, the Ukrainian government is committed to restoring the lost medical infrastructure, and therefore the medical market needs more medical supplies than ever before, from bandages to medical equipment.
All of the above indicates that the medical market is open to global manufacturers of medical products of various kinds.
In particular, the market of laboratory tests, as exemplified by blood collection systems, is represented in Ukraine by 428 centers where blood is donated and stored, including 43 blood centers of regional and city significance; 309 transfusion departments; and 76 hospitals where donors are received. This medical infrastructure requires significant volumes of various consumables, as well as vacuum tubes for blood collection, needles for blood collection, containers for transportation and storage of blood, disinfectants, gloves and clothing for medical personnel, etc.
Therefore, the certificate of conformity during martial law in Ukraine continues to be issued by the designated conformity assessment bodies and is the result of the conformity assessment procedure for medical devices through a remote audit of the manufacturer.