Batch certification is one of the main legal procedures. It can be applied for registration of imported medical devices in Ukraine.
The certificate of conformity for the batch is issued without experts going to the place of production. In fact, it is issued on the basis of a valid technical file and inspection of the lot by an auditor at customs.
Therefore, the procedure for carrying out the procedure is reduced to:
– acquaintance with the technical file;
– preparation of instructions and labeling under Ukrainian legislation;
– drawing up a contract for the customer or Authorized Representative;
– audit of the batch directly at customs;
– certificate issuance (approximately 3 – 5 days);
– customs clearance as a medical product (with a tax rate of 7%).
Obtaining a certificate of conformity for a batch of medical goods is economically feasible if the products are not imported systematically or once. As a rule, this is the supply of artificial medical electrical equipment or medical products of the same type (one model and one brand).
Would you like to learn about the possibility of batch certification of medical products?
Order a preliminary consultation from Miasphera by +38 068 498 88 58
The Lviv Medical Forum 2024 has ended, and we would like to share our results and impressions. The event brought together more than 60 participating companies from Ukraine and Europe. They presented modern medical, dental and rehabilitation equipment, medical and laboratory products, food supplements and latest treatment methods.
Miasphera’s team carefully prepared for the event and was incredibly pleased to become a participant and official partner of such a large-scale event. Useful acquaintances, business discussions, a lot of new information and, of course, a bit of charming old Lviv in our free time from work awaited us.
As part of the GalMED exhibition, Miasphera organized a scientific and practical conference about the legalization of medical cannabis and the certification of medical products. We involved doctors, lawyers, pharmacists and specialists related to the medical industry in the professional discussion.
The topic of medical cannabis caused a lively discussion among the guests. It indicates the great relevance of this issue. We believe this focus on the use of cannabis for medical purposes will find even more interest among the professional medical community in Ukraine.
Miasphera is grateful to everyone who took an active part in the conference and shared the idea of legalization and the potential of using cannabis for medical purposes. Also, we are immensely grateful to the organizers, partners and our Armed Forces for the opportunity to participate in such an event!
And finally, we traditionally present a small photo report.
























There is traditionally a wide range of dietary supplements on the market of Ukraine. They cover many directions of physiological action and have different forms of release. But sometimes, the information on the package about the properties of dietary supplements can confuse consumers, especially if these products were bought in pharmacies.
Therefore, the manufacturer of dietary supplements should pay special attention to labeling. According to the requirements of Ukrainian legislation, all mandatory information about the product must be provided directly on the package or label.
So, a correctly labeled dietary supplement for the manufacturer is not only a powerful incentive to enable the consumer to make an informed choice without misleading him, but also a clear compliance with the requirements of Ukrainian legislation and a minimization of appeals from control authorities.
Understanding the intricacies of medical device certification can seem difficult even for those who have already encountered this topic many times. Therefore, we are sharing useful information and considering the main aspects and issues related to the certification of medical devices on the territory of Ukraine.
Let’s go!
Certification of medical devices is a process of assessing the compliance of a medical device with the requirements of the Technical Regulations. Certification confirms the safety and effectiveness of medical devices used by humans.
Of course! Compliance assessment of medical devices is a legal requirement. This confirms the legal introduction of medical products into circulation on the territory of Ukraine and their subsequent sale.
The responsibility for declaring the medical product lies directly with the manufacturer or an authorized person, in case the medical products are imported from other countries. The declaration is made under the full responsibility of the manufacturer.
The procedure for evaluating the conformity of medical devices depends on the type and safety class of the medical device. The main procedures are self-declaration (for medical devices of the I-risk class, non-sterile, without a measurement function, for in vitro) and assessment of conformity through the manufacturer’s audit (I-sterile, with a measurement function, IIa, IIb, III).
In some cases, the batch certification or the procedure with EU recognition of the certificate is used.
The centers of regulation and supervision are the Ministry of Economic Development and Trade, the Ministry of Health and the State Medical Service of Ukraine.
Assessment of the conformity of medical products in Ukraine is carried out by specially accredited conformity assessment organizations of state or private ownership. The current list of accredited organizations is published by the Ministry of Economic Development and Trade.
Our experts accompany the manufacturer at all stages of the conformity assessment procedure, starting from consultation and collecting documents and ending with obtaining expert opinions. We also provide full legal support and Authorized Representative services.
Have additional questions? Leave a request for a consultation or call us.
We are always in touch +38 068 498 88 58
Have you thought about who is responsible for the quality and safety of imported goods? In Ukraine, this role belongs to the Authorized Representative. This is a legal entity or an individual entrepreneur who has residency.
The presence of an authorized representative for any non-resident manufacturer is a requirement of Ukrainian legislation. Therefore, one of the first steps in the process of certification of medical products or cosmetics is the appointment of the authorized representative of the manufacturer in Ukraine. The authorized representative acts as an official intermediary between the producer and the Ukrainian market, including with state institutions responsible for market surveillance.
An Authorized Representative has certain duties. These duties are defined by the Technical Regulations and legislative acts:
Usually, foreign manufacturers choose an authorized representative according to their own requirements and preferences. This can be an existing distributor or an independent company such as Miasphera.
Let’s consider both cases in more detail.
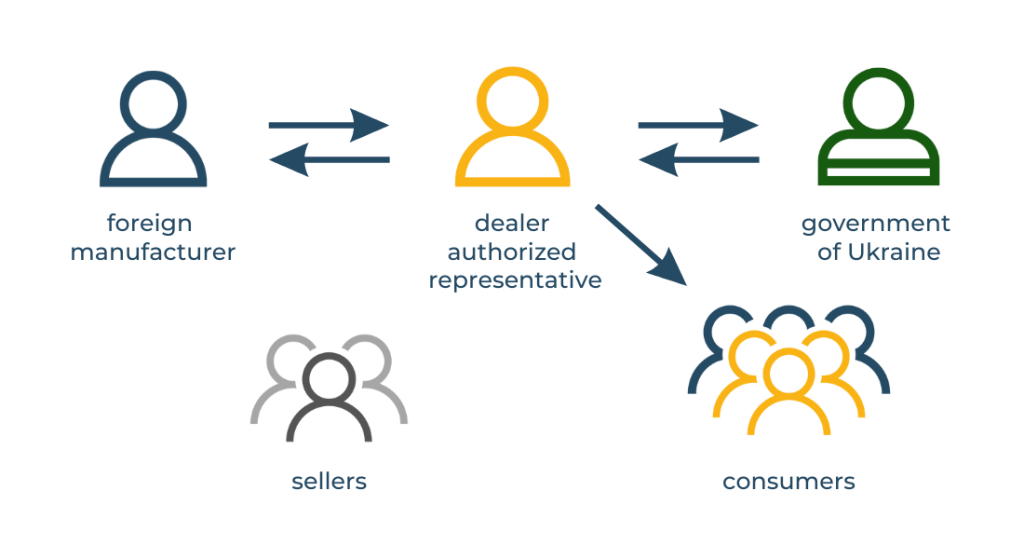
If the authorized representative is a dealer-importer, the manufacturer is more dependent on its conditions. In this case, the representative-dealer often acts in his own interests, closing the supply and sales to himself and limiting the rights of the dealership to other companies. In addition, he has an additional competitive advantage – his name, as an Authorized Representative, is present on the labeling or on the instructions of all products.

If the Authorized Representative is a company that is not involved in the sale of products, the manufacturer can independently choose any number of dealers with whom he wants to cooperate.
Important clarification! Each type of medical device must be associated with only one Authorized Representative.
As you can see, the last option has more advantages for the manufacturer, as it allows you to regulate the number of dealers and grant the right to import and distribute your products to other companies.
Miasphera offers independent outsourcing services of the Authorized Representation, because we have many years of experience.
We work with a wide range of medical, cosmetic, food and veterinary products and always keep our finger on the pulse of legislative innovations.
Contact us and take an important step towards a successful start on the Ukrainian market!
On March 12–14 in Kyiv, the Pro Beauty Expo 2024 was held. These unforgettable three days of the exhibition have passed very quickly.
Miasphera was pleasantly surprised by the coordinated organization and incredibly stylish stands of the participating companies, which left a pleasant aftertaste of aesthetic satisfaction.
Our team received a dose of spring inspiration, a charge of energy and, of course, many new pleasant acquaintances and contacts in the cosmetology industry.
And so, after catching our breath, incredibly inspired, we start planning visits to the next events in the field of beauty.
See you at the next events. Let’s see how it was 🙂





















The use of cannabis in the world for any purpose has always been the subject of intense attention among medical professionals and public opinion. And our country is no exception! The stages of legalization of medical cannabis in Ukraine are still going through a thorny path of legislative vicissitudes and changes.
But in the last year, the issue of the legal status of cannabis and its legalization in Ukraine has made significant progress.
An important event took place on February 13, 2023. The President of Ukraine signed Law №7457 on regulating the circulation of medical cannabis. The law will enter into force six months after its promulgation.
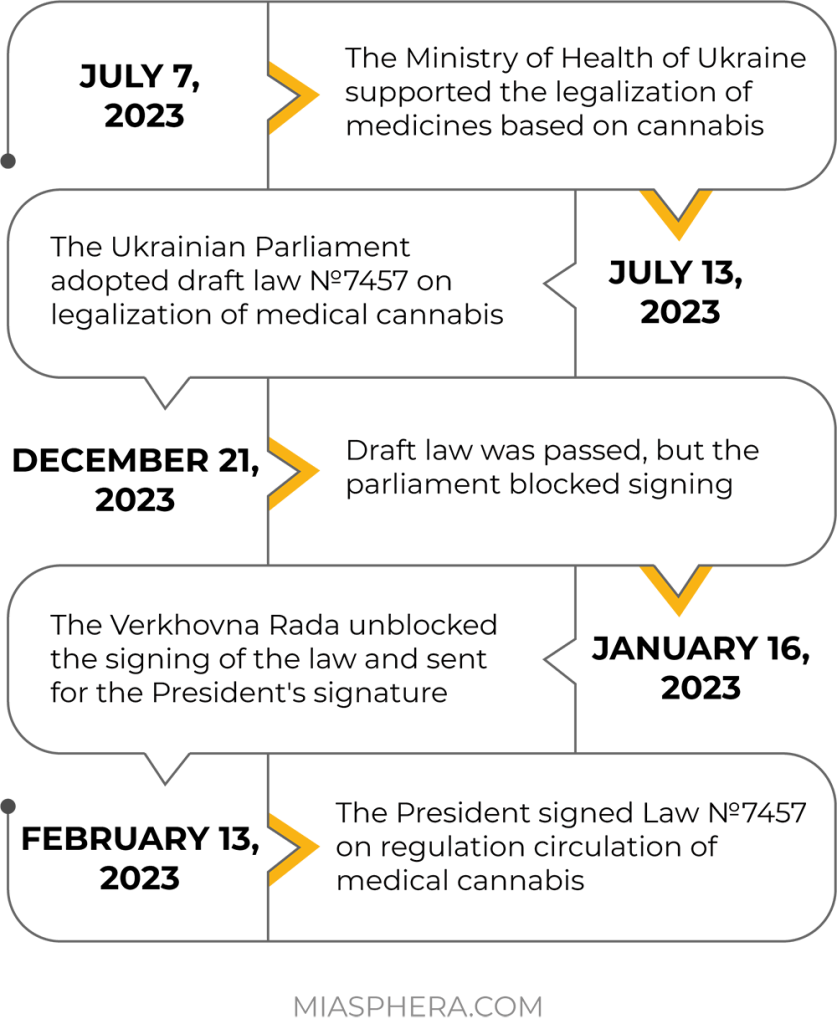
The main goal of the law is to increase access to medical cannabis preparations for the treatment of a number of diseases. According to the Ministry of Health, more than 2 million Ukrainians currently need cannabis-based drugs. The adopted law declares that the circulation of varieties of plants of the genus hemp (Cannabis) for medical purposes, products of their processing and medicines produced from them, is allowed only for the purpose of their use in medical, scientific and scientific and technical practice.
Distribution and consumption of cannabis for recreational purposes among the public will be prohibited. Therefore, a potentially important new tool can be considered the introduction of a traceability system for the circulation of varieties of hemp plants.
The issue of cannabis legalization in Ukraine opens up a wide space for discussion of new business prospects. This can be a significant stimulus for the development of new sectors of the economy, not only in the field of the production of medical and cosmetic preparations based on Cannabis plants, but also the industrial cultivation and processing of hemp. Further scientific research into the properties of existing varieties of hemp and their subspecies is also of interest.
In one case or another, the adoption of the law does not mean that cannabis plants and drugs based on them will be widely available to Ukraine already this year. We can safely predict that some time after the adoption of the law, the electronic registers defined by the draft law will be formed, algorithms for controlling the circulation of plants, conditions for granting quotas, etc. will be prescribed.

Vitaliy Kovbasa, a leading lawyer at the Miasphera consulting company, talks about the prospects and conditions for the legalization of cannabis-related activities in Ukraine.
“Rejecting skepticism and assessing the situation objectively, we can state this process will not be clearly defined in the nearest time. Those who wish to pursue cannabis-related activities in the future need to worry about the legal side now. The most important thing before starting such activities is to ensure the availability of appropriate licenses and pass the appropriate certification procedures,” Vitaliy comments.
What should be taken into account?
Licensing, namely obtaining a license for:
Certification, namely:
Therefore, all types of activities related to the circulation of hemp plants are allowed only if the subjects of such activities have the appropriate licenses and undergo the appropriate certification procedures.
Disclaimer: This article doesn’t promote the use of drugs or any other illegal substances. The text is a description of the history and scientific studies of the influence of cannabis on society, man, and his physical and mental health.
Dear customers and partners!
We have important news for you. Miasphera has joined the Association of Medical Devices Market Operators (AMOMD).
The Association of Medical Devices Market Operators is a Ukrainian professional association that includes about 80 national manufacturers, importers, distributors of medical devices, cosmetic products, personal protective equipment, and disinfectants.
The main mission of the association is to promote the development and legal protection of the interests of all participants in this market. Such consolidation makes it possible to maintain healthy competition in the industry, influence legislative regulation and protect the professional interests of participants before the state.
Currently, Miasphera actively promotes its services specifically for national manufacturers of medical products. Therefore, cooperation within the Association is an important step towards dialogue and the establishment of new partnership relations.
The mission of Miasphera is to help businesses develop within the limits of legislative norms on the territory of Ukraine, thus making a significant contribution to the development of the country’s medical industry.

The first mentions of the cultivation of cannabis by mankind date back to 4000 BC. This plant has been used for thousands of years as a medicine, as a source of food, fiber, oil and paper.
The history of mankind’s interrelations with cannabis has always been influenced by various cultural, social and local trends. Cannabis was used to treat various diseases in ancient China and India. Cannabis was used in the treatment of constipation, malaria, rheumatic pains and women’s ailments. The one spread to Africa and Arab countries in the Middle Ages, where it was used mostly in medicine, as well as in cultural and religious rituals. Marijuana was first used as a pain reliever in Ancient Greece. Cannabis spread to Europe and America in the 15th century, where its use became especially popular among doctors and artists.
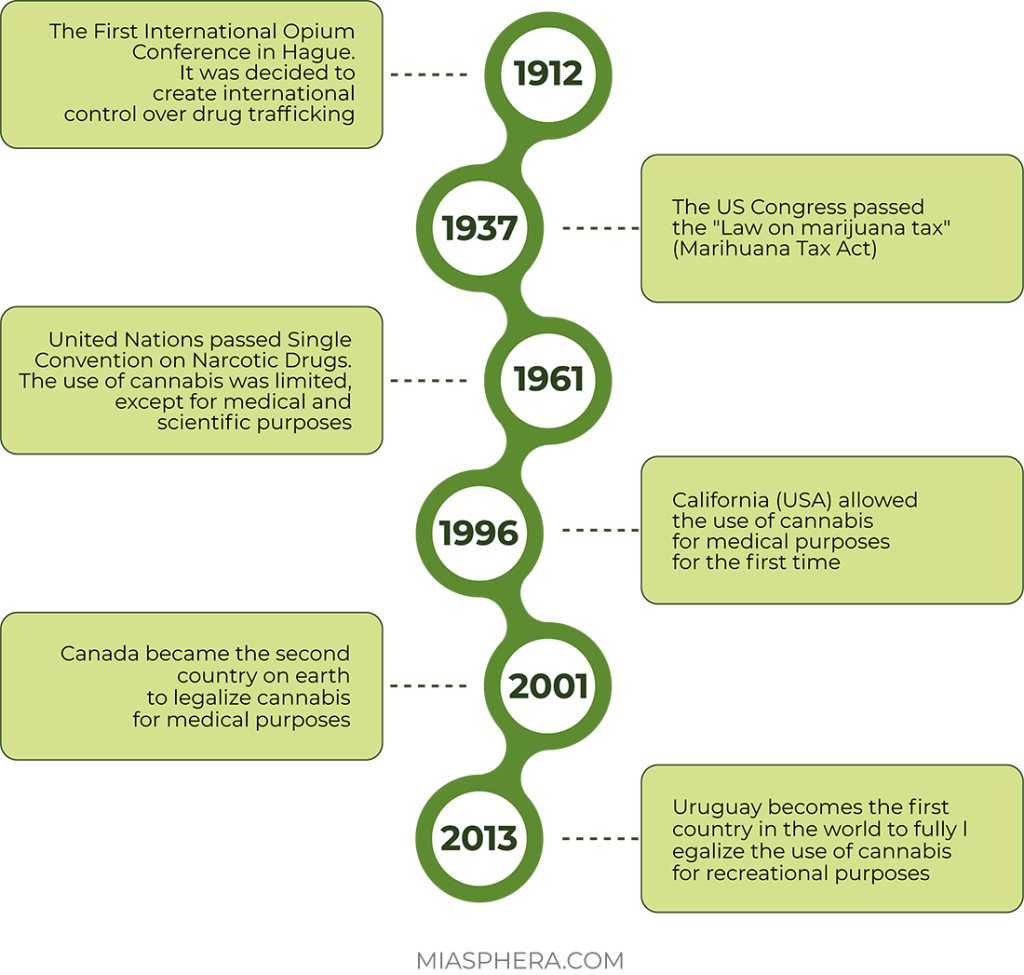
At the beginning of the 20th century, there was a rapid growth in the consumption of cannabis, including due to its recreational properties. At the same time, despite its great popularity, cannabis began to be banned en masse. It all started with the international opium convention signed in 1912. Initial control of the circulation of hard drugs and cannabis was introduced between the participating countries: Germany, Prussia, the Russian Empire, Great Britain, the Netherlands and many others. Marijuana was allowed to be produced and used only for medical purposes.
During the 1930s, an anti-marijuana campaign arose in the United States. The cannabis tax law was introduced in 1937.
At the same time, the popularity of hemp continued to grow in industry, for example for the production of ropes, shoes and clothing. In 1942, Henry Ford creates an experimental body made of hemp, which turned out to be 10 times stronger than steel of the same thickness. Hemp biodiesel was supposed to be the fuel for the car.
In the 1960s and 1970s, marijuana became especially popular among the counterculture youth, who became its main consumers. It becomes a kind of symbol of the hippie movement, as well as musical and artistic self-expression.
During this period, cannabis becomes the object of prohibition in many countries due to the danger of abuse and its psychotropic properties. The world history of marijuana takes a new turn in March 1961. The UN adopted the Single Convention on Narcotic Drugs. According to the cannabis convention, cannabis resin, cannabis extracts and tinctures were included in the list of narcotic drugs. The signatory countries were obliged to strengthen control over the cultivation of the plant, and to completely stop its use for purposes other than medical and scientific. Cultivation of industrial varieties of cannabis did not fall under the ban.
Many countries began the process of legalizing cannabis for medical and recreational purposes already at the end of the 20th century. It was an example of breaking traditional stereotypes.
Legal access to herbal cannabis and its use for medical purposes under the supervision of a doctor was first allowed in the state of California (USA) in 1996. In 2001, Canada became the second country in the world to legalize the use of cannabis for medical purposes. Since October 17, 2018, the use of cannabis for medical purposes has been allowed.
On December 10, 2013, the Uruguayan Senate passed a law on the complete decriminalization of the cultivation, sale, purchase and use of marijuana. Uruguay became the first country in the world to fully legalize cannabis.
In December 2020, the UN Commission on Narcotic Drugs removed medical cannabis and all its derivatives from the list of critically dangerous drugs, but about 25 countries still voted against this initiative.
Currently, the world market of cannabis, including for medical purposes, is in the process of rapid development. However, this development is not uniform due to different legal environments and mixed public attitudes.
In contrast to the legalization of recreational use of cannabis, more and more countries allow its medical use every year. In the last 5 years, about 20 countries in Europe (Greece, Estonia, Ireland, Luxembourg, Malta, Macedonia, Germany, Norway, Poland, Portugal, Croatia), North and South America (Argentina, Colombia, Mexico, Peru, Chile) and other countries (Australia, Philippines, Jamaica) have legalized cannabis and cannabinoids for medical purposes.
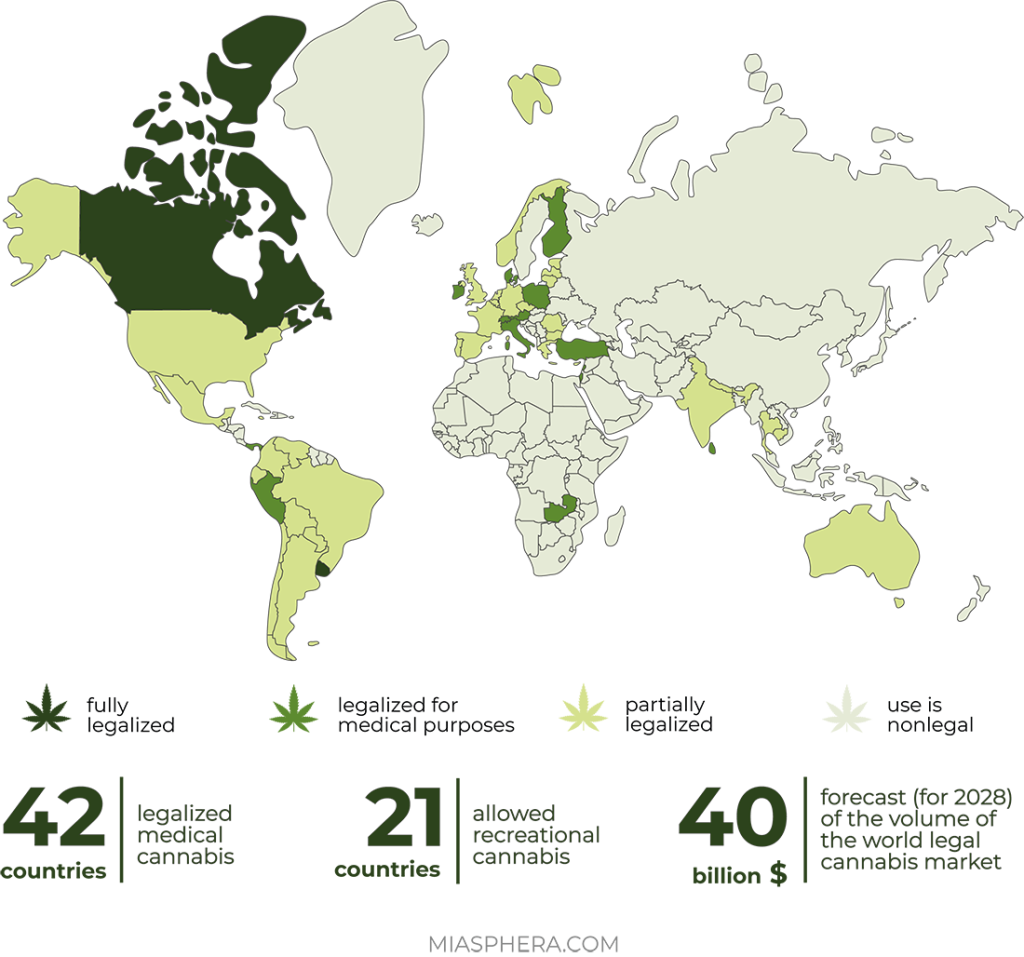
Some countries, such as Canada and the US, have adopted fully regulated frameworks that allow the cultivation, consumption and retail distribution of various cannabis-based products. So in South Africa, it is legally allowed to grow hemp, but not legalized retail sales.
In general, despite the slow changes, the world is witnessing the liberalization of the legal framework of international documents regarding the circulation of cannabis compounds and cannabinoids for medical purposes and the decriminalization of their use. Such drugs and preparations are gradually becoming available to patients in different countries.
Medical devices are classified depending on the design, features of application and potential danger in case of their incorrect use. Therefore, before starting the certification process, the manufacturer of the medical device must determine its medical purpose and intended use.
Each country has its own requirements. Currently, the medical device classifier NC 024:2023 operates in Ukraine. It is created on the basis of the GMDN international nomenclature.
The safety class of a medical product depends on the potential risk during its use by the consumer. Medical products are classified according to the following criteria: invasiveness, duration of use, presence of contact with the human body, effect on vital human organs, as well as the possibility of using energy sources together with the product.
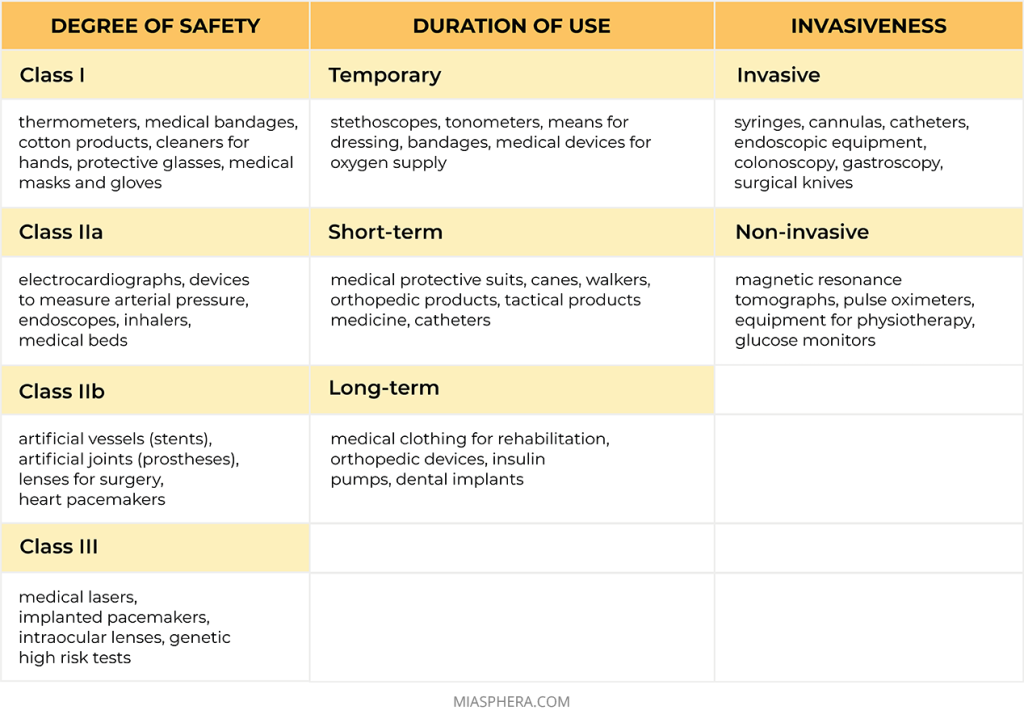
So, according to the degree of safety, medical products are divided into:
Class I — a low proportion of risk
Class IIa — the average proportion of risk
Class IIb — increased risk
Class III — high proportion of risk
According to the duration of use, medical products are:
Temporary – for continuous use up to 60 minutes;
Short-term – for continuous use up to 30 days;
Long-term – for continuous use for a period of 30 days or more.
According to invasiveness, medical products are:
Non-invasive are medical devices or means that do not require invasion or penetration into the human body for diagnosis, monitoring or treatment.
Invasive are medical products that are completely or partially introduced into the human body through its surface or body opening.
Accepted safety classes of medical devices reflect the potential risk in case of their use by consumers. Therefore, depending on the defined class and characteristics, the medical device certification procedure may differ. After all, the higher the risk class, the more complicated the registration procedure is.
In the further sale of products, mistakes in determining the class can be expensive. Therefore, if you have doubts about the correct classification of your own product, contact Miasphera specialists!
We will be happy to provide comprehensive advice and suggest the best certification options for your product.
Leave an application for consultation.