
Today, every global medical device manufacturer is undoubtedly facing a difficult question: “Should I import medical devices to Ukraine?” And the answer is very simple: “Yes, of course”.
For many global medical device manufacturers that were present on the Ukrainian market before the war, the current permits for medical devices are now a stellar time, a time when Ukraine is gradually rebuilding and restoring its damaged medical infrastructure.
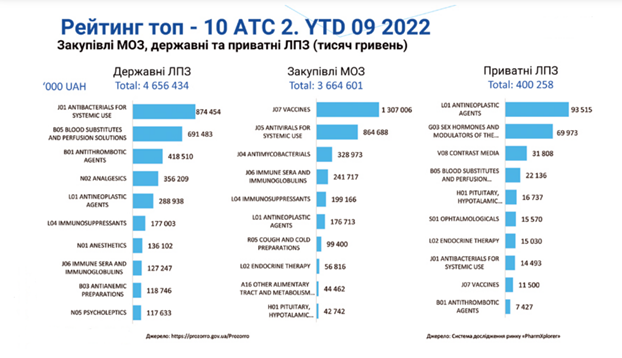
To support the positive answer to the above question regarding the import of medical devices to Ukraine, we provide statistical data:
The state budget of Ukraine for 2022 allocates UAH 195.9 billion for healthcare, and with additional funding due to the full-scale war – UAH 232.8 billion. In 2023, the budget allocates UAH 175 billion for healthcare, including UAH 10.3 billion for centralized procurement of medical products in 31 areas in 2023.
However, Ukraine is on a course to standardize with Europe, and while restoring and rebuilding the lost medical infrastructure, it aims to introduce the latest technologies and products, including medical devices.
Therefore, it is time for global manufacturers with the latest and most advanced medical technologies and products to discover the Ukrainian market and start importing medical devices to Ukraine.
An Authorized Representative appointed in Ukraine will help a global manufacturer of medical devices to overcome all the difficulties related to the import of medical devices to Ukraine. Since any import of medical devices to Ukraine for manufacturers of medical devices that are not residents of Ukraine is not possible without the appointment of an Authorized Representative in Ukraine.
It is the Authorized Representative, who may be a trade participant or solely the holder of registration documents for a medical device, who takes all the necessary actions to ensure that the import of medical devices to Ukraine is successful.
Importation requires a number of mandatory actions and additional actions at the choice of the medical device manufacturer. It is mandatory to conclude an agreement with a company from Ukraine on the provision of services of an Authorized Representative.
Having confirmation of his/her authority, the Authorized Representative examines the technical documentation for the medical device in order to establish the necessary procedure for assessing the conformity of the medical device and subsequently initiates its implementation. In other words, import of medical devices to Ukraine that are class I medical devices, non-sterile, without measurement function and medical devices for in vitro diagnostics, and not intended for self-monitoring is possible upon registration of the manufacturer and the Authorized Representative in the Register of the State Service of Ukraine on Medicines and Drugs Control. Instead, medical devices of a higher risk class are subject to the manufacturer’s audit procedure with the involvement of the designated medical device conformity assessment body.
Having such documents as a declaration of conformity of medical devices and/or a certificate of conformity assessment for a medical device, the most difficult stages before importation are completed.
Ukrainian legislation allows importing medical devices into Ukraine subject to confirmation of their compliance with the Technical Regulations (as evidenced by the aforementioned declaration and/or certificate), the label for the medical device developed in Ukrainian, and the instruction for use of the medical device.
Due to the fact that in Ukraine, as in other countries, the circulation of medical devices is regulated, a number of requirements are set for the said labels and instructions for use of the medical device in terms of their form and content. This complex task is undertaken by the Authorized Representative, and the medical device manufacturer will only need to provide technical files for the medical device, which will be processed by the Authorized Representative to develop the correct label and instruction for the medical device.
The above-mentioned documents are subject to inspection at the customs during the regulated procedures for customs clearance of medical devices in Ukraine, which are determined depending on the medical device, according to the UKTZED code for the product.
From this stage, the manufacturer of medical devices can carry out the production of such devices in order to import medical devices to Ukraine. And the benefit of the Authorized Representative at the following stages is no less important, since the Authorized Representative can search for a logistics partner for the import of goods, advise a reliable dealer, and take actions aimed at preventing gray imports of medical devices by the manufacturer in the Ukrainian market. The above actions are additional for the medical device manufacturer, but no less important for the successful import and sale of medical devices in the Ukrainian market.
Importing medical devices to Ukraine is simple, not expensive and predictably successful if you entrust this business to a reliable partner – an Authorized Representative.
Import medical devices to Ukraine – join the rebuilding and restoration of Ukraine, for peace, justice and democratic values.