
Medical devices are classified depending on the design, features of application and potential danger in case of their incorrect use. Therefore, before starting the certification process, the manufacturer of the medical device must determine its medical purpose and intended use.
Each country has its own requirements. Currently, the medical device classifier NC 024:2023 operates in Ukraine. It is created on the basis of the GMDN international nomenclature.
The safety class of a medical product depends on the potential risk during its use by the consumer. Medical products are classified according to the following criteria: invasiveness, duration of use, presence of contact with the human body, effect on vital human organs, as well as the possibility of using energy sources together with the product.
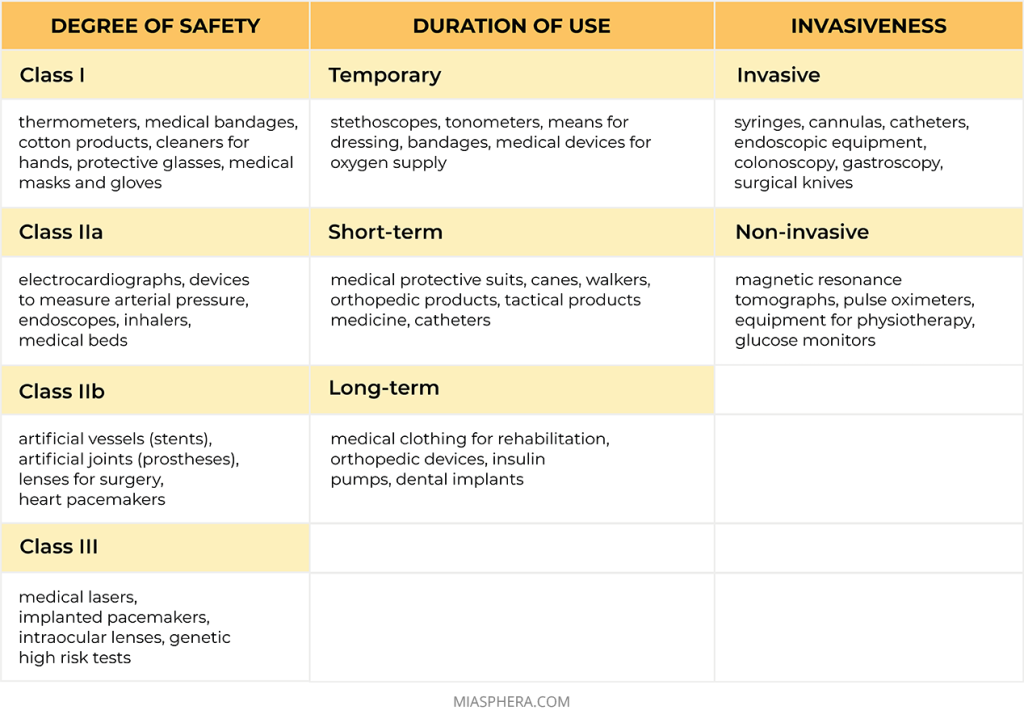
So, according to the degree of safety, medical products are divided into:
Class I — a low proportion of risk
Class IIa — the average proportion of risk
Class IIb — increased risk
Class III — high proportion of risk
According to the duration of use, medical products are:
Temporary – for continuous use up to 60 minutes;
Short-term – for continuous use up to 30 days;
Long-term – for continuous use for a period of 30 days or more.
According to invasiveness, medical products are:
Non-invasive are medical devices or means that do not require invasion or penetration into the human body for diagnosis, monitoring or treatment.
Invasive are medical products that are completely or partially introduced into the human body through its surface or body opening.
Accepted safety classes of medical devices reflect the potential risk in case of their use by consumers. Therefore, depending on the defined class and characteristics, the medical device certification procedure may differ. After all, the higher the risk class, the more complicated the registration procedure is.
In the further sale of products, mistakes in determining the class can be expensive. Therefore, if you have doubts about the correct classification of your own product, contact Miasphera specialists!
We will be happy to provide comprehensive advice and suggest the best certification options for your product.
Leave an application for consultation.